Abstract
Background and aims
The major cause of Hepatocellular carcinoma (HCC) is acute or chronic infection caused by hepatotropic viruses and HBV infection is the main cause. UHRF2, a ubiquitin-protein ligase E3, is associated with cancer development. This study aimed to investigate the connection and mechanism between UHRF2 and HBV-associated HCC.
Methods
The expression of UHRF2 in human HBV-positive HCC tissues and paracancerous tissues was detected by western blot and tissue microarray. The effects of UHRF2 on invasion, migration and proliferation were detected in HBV-positive hepatoma cell lines. Furthermore, western blot, immunofluorescence, Co-immunoprecipitation and ubiquitination assays were used to explore the relationship and mechanism between UHRF2 and HBV-associated HCC.
Results
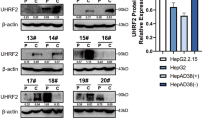
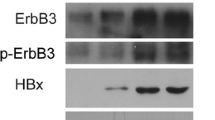
HBV-positive HCC tissues had higher UHRF2 expression levels than adjacent non-tumor tissues. The HBV-positive HCC patients with a low UHRF2 level in cancer tissues had longer overall and recurrence-free survival compared with those with a high UHRF2 level. UHRF2 induced invasion, migration and proliferation in human HBV-positive HCC cell lines HepG2.2.15 and Hep AD38(−). HBx, an encoding protein of HBV, maintained the stability of UHRF2 by blocking the ubiquitination of UHRF2. HBx up-regulated CDK2 expression through ETS1. UHRF2 bound to CDK2 directly and enhanced UHRF2 phosphorylation at serine 643.
Conclusions
These results suggest that HBx-ETS1-CDK2-UHRF2 pathway plays an important role in the pathogenesis of HBV-associated HCC and represents new therapeutic targets for human HCC.
Clinical Trials Registration
ChiCTR2000041416.






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424
Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol Res 2015;45(1):59–74
Petr U, Petr H. Hepatocellular carcinoma from the view of gastroenterologist/hepatologist. Klin Onkol 2020;33(Supplementum 3):34–44
Ng S-A, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol 2011;46(8):974–990
Du Y, Kong G, You X, Zhang S, Zhang T, Gao Y, et al. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem 2012;287(31):26302–26311
Qian G, Jin F, Chang L, Yang Y, Peng H, Duan C. NIRF, a novel ubiquitin ligase, interacts with hepatitis B virus core protein and promotes its degradation. Biotech Lett 2012;34(1):29–36
Bronner C, Achour M, Arima Y, Chataigneau T, Saya H, Schini-Kerth VB. The UHRF family: oncogenes that are drugable targets for cancer therapy in the near future? Pharmacol Ther 2007;115(3):419–434
Wang F, Zhang P, Ma Y, Yang J, Moyer MP, Shi C, et al. NIRF is frequently upregulated in colorectal cancer and its oncogenicity can be suppressed by let-7a microRNA. Cancer Lett 2012;314(2):223–231
Lu H, Hallstrom TC. The nuclear protein UHRF2 is a direct target of the transcription factor E2F1 in the induction of apoptosis. J Biol Chem 2013;288(33):23833–23843
Wang Y, Yan X, Zeng S, Zhang T, Cheng F, Chen R, et al. UHRF2 promotes DNA damage response by decreasing p21 via RING finger domain. Biotech Lett 2018;40(8):1181–1188
Wu J, Liu S, Liu G, Dombkowski A, Abrams J, Martin-Trevino R, et al. Identification and functional analysis of 9p24 amplified genes in human breast cancer. Oncogene 2012;31(3):333–341
Zeng S, Wang Y, Zhang T, Bai L, Wang Y, Duan C. E3 ligase UHRF2 stabilizes the acetyltransferase TIP60 and regulates H3K9ac and H3K14ac via RING finger domain. Protein Cell 2017;8(3):202–218
Lu S, Yan D, Wu Z, Jiang T, Chen J, Yuan L, et al. Ubiquitin-like with PHD and ring finger domains 2 is a predictor of survival and a potential therapeutic target in colon cancer. Oncol Rep 2014;31(4):1802–1810
Jin C, Xiong D, Li H-R, Jiang J-H, Qi J-C, Ding J-Y. Loss of UHRF2 Is associated with non-small cell lung carcinoma progression. J Cancer 2018;9(17):2994–3005
Lempp FA, Mutz P, Lipps C, Wirth D, Bartenschlager R, Urban S. Evidence that hepatitis B virus replication in mouse cells is limited by the lack of a host cell dependency factor. J Hepatol 2016;64(3):556–564
Ren JH, Hu JL, Cheng ST, Yu HB, Wong VKW, Law BYK, et al. SIRT3 restricts hepatitis B virus transcription and replication through epigenetic regulation of covalently closed circular DNA involving suppressor of variegation 3–9 homolog 1 and SET domain containing 1A histone methyltransferases. Hepatology 2018;68(4):1260–1276
Li Y, Mori T, Hata H, Homma Y, Kochi H. NIRF induces G1 arrest and associates with Cdk2. Biochem Biophys Res Commun 2004;319(2):464–468
Kaul R, Risinger AL, Mooberry SL. Eribulin rapidly inhibits TGF-beta-induced Snail expression and can induce slug expression in a Smad4-dependent manner. Br J Cancer 2019;121(7):611–621
Takase N, Koma Y, Urakawa N, Nishio M, Arai N, Akiyama H, et al. NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett 2016;380(1):47–58
Zhang F, Yang Q, Meng F, Shi H, Li H, Liang Y, et al. Astrocyte elevated gene-1 interacts with β-catenin and increases migration and invasion of colorectal carcinoma. Mol Carcinog 2013;52(8):603–610
Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschläger M, et al. In vitro cell migration and invasion assays. Mutat Res Rev Mutat Res 2013;752(1):10–24
Singh AK, Swarnalatha M, Kumar V. c-ETS1 facilitates G1/S-phase transition by up-regulating cyclin E and CDK2 genes and cooperates with hepatitis B virus X protein for their deregulation. J Biol Chem 2011;286(25):21961–21970
Qin D, Li K, Qu J, Wang S, Zou C, Sheng Y, et al. HBx and HBs regulate RhoC expression by upregulating transcription factor Ets-1. Adv Virol 2013;158(8):1773–1781
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 2017;16:1
Han LL, Lv Y, Guo H, Ruan ZP, Nan KJ. Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy. World J Gastroenterol 2014;20(30):10249–10261
Mori T, Li Y, Hata H, Ono K, Kochi H. NIRF, a novel RING finger protein, is involved in cell-cycle regulation. Biochem Biophys Res Commun 2002;296(3):530–536
Iguchi T, Ueda M, Masuda T, Nambara S, Kidogami S, Komatsu H, et al. Identification of UHRF2 as a negative regulator of epithelial-mesenchymal transition and its clinical significance in esophageal squamous cell carcinoma. Oncology 2018;95(3):179–187
Lu HR, Bhoopatiraju S, Wang HB, Schmitz NP, Wang XH, Freeman MJ, et al. Loss of UHRF2 expression is associated with human neoplasia, promoter hypermethylation, decreased 5-hydroxymethylcytosine, and high proliferative activity. Oncotarget 2016;7(46):76047–76061
Peng R, Huang X, Zhang C, Yang X, Xu Y, Bai D. Overexpression of UHRF2 in intrahepatic cholangiocarcinoma and its clinical significance. OncoTargets Ther 2017;10:5863–5872
Lai M, Liang L, Chen J, Qiu N, Ge S, Ji S, et al. Multidimensional proteomics reveals a role of UHRF2 in the regulation of epithelial-mesenchymal transition (EMT). Mol Cell Proteom 2016;15(7):2263–2278
Li L, Duan QH, Zeng ZY, Zhao JD, Lu JW, Sun JL, et al. UHRF2 promotes intestinal tumorigenesis through stabilization of TCF4 mediated Wnt/beta-catenin signaling. Int J Cancer 2020;147(8):2239–2252
He X, Duan C, Chen J, Ou-Yang X, Zhang Z, Li C, et al. Let-7a elevates p21(WAF1) levels by targeting of NIRF and suppresses the growth of A549 lung cancer cells. FEBS Lett 2009;583(21):3501–3507
Wu TF, Zhang W, Su ZP, Chen SS, Chen GL, Wei YX, et al. UHRF2 mRNA expression is low in malignant glioma but silencing inhibits the growth of U251 glioma cells in vitro. Asian Pac J Cancer Prev 2012;13(10):5137–5142
Liu N, Zhang J, Yang X, Jiao T, Zhao X, Li W, et al. HDM2 pmotes NEDDylation of Hepatitis B virus HBx to enhance its stability and function. J Virol 2017;91(16):e00340-17
Zhao J, Wang C, Wang J, Yang X, Diao N, Li Q, et al. E3 ubiquitin ligase Siah-1 facilitates poly-ubiquitylation and proteasomal degradation of the hepatitis B viral X protein. FEBS Lett 2011;585(19):2943–2950
Zhao J, Wu J, Cai H, Wang D, Yu L, Zhang W-H. E3 ubiquitin ligase Siah-1 is down-regulated and fails to target natural HBx truncates for degradation in hepatocellular carcinoma. J Cancer 2016;7(4):418–426
Zhang H, Huang C, Wang Y, Lu Z, Zhuang N, Zhao D, et al. Hepatitis B virus X protein sensitizes TRAIL-induced hepatocyte apoptosis by inhibiting the E3 ubiquitin ligase A20. PLoS One 2015;10(5):e0127329
Wang J, Li N, Huang Z-B, Fu S, Yu S-M, Fu Y-M, et al. HBx regulates transcription factor PAX8 stabilization to promote the progression of hepatocellular carcinoma. Oncogene 2019;38(40):6696–6710
Minor MM, Hollinger FB, McNees AL, Jung SY, Jain A, Hyser JM, et al. Hepatitis B virus HBx protein mediates the degradation of host restriction factors through the Cullin 4 DDB1 E3 ubiquitin ligase complex. Cells 2020;9(4):834
Yeom S, Kim SS, Jeong H, Jang KL. Hepatitis B virus X protein activates E3 ubiquitin ligase Siah-1 to control virus propagation via a negative feedback loop. J Gen Virol 2017;98(7):1774–1784
Gu Y, Zhang J, Ma X, Kim BW, Wang H, Li J, et al. Stabilization of the c-Myc protein by CAMKIIgamma promotes T cell lymphoma. Cancer Cell 2017;32(1):115 e117–128 e117
Lee S, Kim W, Ko C, Ryu WS. Hepatitis B virus X protein enhances Myc stability by inhibiting SCFSkp2 ubiquitin E3 ligase-mediated Myc ubiquitination and contributes to oncogenesis. Oncogene 2015;35(14):1857–1867
Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S,et al. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat Methods 2013;10(7):676–682
Mukherji A, Janbandhu Vaibhao C, Kumar V. HBx-dependent cell cycle deregulation involves interaction with cyclin E/A–cdk2 complex and destabilization of p27Kip1. Biochem J 2006;401(1):247–256
Acknowledgements
We thank Dr. Rui Yang and Yan Sun for their technical supports.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81772178) and Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University (2019).
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. FC and GQ contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
Fengjuan Cheng, Guanhua Qian, Xianyun Fang, Jingjie Sun, Siyuan Chen, Rongjuan Chen, Shangjing Liu, Zhaodi Li, Kejia Wu, Shiming Jiang, Yong Chen, Ni Tang, Juan Chen, and Changzhu Duan declare that they have no conflict of interest.
Ethics approval
This study was performed in accordance with the declaration of Helsinki. HBV-positive HCC tissue samples and paired adjacent normal tissues were collected from the First Affiliated Hospital of Chongqing Medical University. Usage of these patient tissue samples was approved by the Ethics Committee of Chongqing Medical University (committee’s reference number: 2018027).
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, F., Qian, G., Fang, X. et al. HBx promotes hepatocarcinogenesis by enhancing phosphorylation and blocking ubiquitinylation of UHRF2. Hepatol Int 15, 707–719 (2021). https://doi.org/10.1007/s12072-021-10172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10172-z