Abstract
Background
Among elder children/young adults who received hepatitis B virus (HBV) vaccination during infancy, the serological status of HBsAg-negative and anti-HBc-positive [HBsAg(–)/anti-HBc(+)] was frequently reported, indicating potential occult HBV infection (OBI). It is required to define the long-term protection of neonatal vaccination against OBI in their mature adulthood.
Methods
Building upon the 1983–1990 established Qidong Hepatitis B Intervention Study, we sampled 10% of the 28–35-year-old participants, who remained in the cohort by 2012. Each participant was tested for serological markers of HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc. HBV-DNA and relaxed circular DNA (rcDNA) were determined in some HBsAg(–)/anti-HBc(+) individuals.
Results
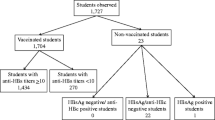
Totally, 3615 individuals from the neonatal vaccination group and 3100 individuals from the control group donated blood samples, respectively. In the vaccination group, the prevalence of HBsAg was 1.58% (57/3615), HBsAg(–)/anti-HBc(+) was 4.70% (170/3615), significantly lower than in the control group, which was 7.45% (231/3100) and 19.48% (640/3100) respectively (all p < 0.001). With aging, HBsAg(–)/anti-HBc(+) prevalence increased in the sampled participants from the control group (pfor trend < 0.001), but uncertain from the vaccination group. Of HBsAg(–)/anti-HBc(+), HBV-DNA was detected in 13.08% (17/130) from the vaccination group, and in 4.18% (12/287) from the control group. HBV rcDNA was detected in most sera that were tested positive for HBV-DNA.
Conclusions
OBI occurred in some vaccinated adults. However, neonatal HBV vaccination kept the effective protection against OBI in mature adults.
Graphic abstract




Similar content being viewed by others
Data availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available for researchers who meet the criteria for access to confidential data from the Ethics Committee, National Cancer Center /Cancer Hospital, Chinese Academy of Medical Sciences. The administrator at the Office of Science and Research, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences can be contacted to data request by email: keyanchu@cicams.ac.cn and quchf@cicams.ac.cn.
References
Pol S. Hepatitis: HBV vaccine—the first vaccine to prevent cancer. Nat Rev Gastroenterol Hepatol 2015;12:190–191
Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013;310:974–976
Qu C, Chen T, Fan C, Zhan Q, Wang Y, Lu J, et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med 2014;11:e1001774
Cui F, Shen L, Li L, Wang H, Wang F, Bi S, et al. Prevention of chronic hepatitis b after 3 decades of escalating vaccination policy. China Emerg Infect Dis 2017;2017(23):765–772
Zheng R, Qu C, Zhang S, Zeng H, Sun K, Chen W, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res 2018;2018(30):571–579
Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477–191 (e471)
Thomas DL. Global elimination of chronic hepatitis. N Engl J Med 2019;380:2041–2050
Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015;2015(479–480):672–686
Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, et al. Two decades of universal hepatitis B vaccination in Taiwan: impact and implication for future strategies. Gastroenterology 2007;2007(132):1287–1293
Carmody E. Time to re-evaluate the effect of the adolescent booster of hepatitis B vaccine. Int J Infect Dis 2017;2017(60):88–90
Hsu HY, Chang MH, Ni YH, Chiang CL, Wu JF, Chen HL, et al. Chronologic changes in serum hepatitis B virus DNA, genotypes, surface antigen mutants and reverse transcriptase mutants during 25-year nationwide immunization in Taiwan. J Viral Hepat 2017;2017(24):645–653
Yang YT, Huang AL, Zhao Y. The prevalence of hepatitis B core antibody in vaccinated Chinese children: a hospital-based study. Vaccine 2019;37:458–463
Xu L, Wei Y, Chen T, Lu J, Zhu CL, Ni Z, et al. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 2010;28:5986–5992
Mu SC, Lin YM, Jow GM, Chen BF. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol 2009;2009(50):264–272
Wang Y, Chen T, Lu LL, Wang M, Wang D, Yao H, et al. Adolescent booster with hepatitis B virus vaccines decreases HBV infection in high-risk adults. Vaccine 2017;35:1064–1070
Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol 2019;2019(71):397–408
Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014;2014(21):153–162
Liu Y, Zeng W, Xi J, Liu H, Liao H, Yu G, et al. Over-gap PCR amplification to identify presence of replication-competent HBV DNA from integrated HBV DNA: An updated occult HBV infection definition. J Hepatol 2019;2019(70):557–559
Sun Z, Zhu Y, Stjernsward J, Hilleman M, Collins R, Zhen Y, et al. Design and compliance of HBV vaccination trial on newborns to prevent hepatocellular carcinoma and 5-year results of its pilot study. Cancer Detect Prev 1991;1991(15):313–318
Yang S, Wang D, Zhang Y, Yu C, Ren J, Xu K, et al. Transmission of hepatitis B and C virus infection through body piercing: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1893
Allain JP, Opare-Sem O. Screening and diagnosis of HBV in low-income and middle-income countries. Nat Rev Gastroenterol Hepatol 2016;2016(13):643–653
Matsumoto A, Imaizumi M, Tanaka Y, Nishiguchi S, Yatsuhashi H, Ishida T, et al. Novel and highly sensitive immunoassay for total hepatitis B surface antigen, including that complexed with hepatitis B surface antibody. J Gastroenterol 2017;52:376–384
Sadeghi A, Yahyapour Y, Poortahmasebi V, Shahmoradi S, Roggendorf M, Karimzadeh H, et al. Clearance of HBV DNA in immunized children born to HBsAg-positive mothers, years after being diagnosed with occult HBV infection. J Viral Hepat 2016;2016(23):282–285
Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, et al. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J Hepatol 2012;2012(57):720–729
Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, et al. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun 2015;6(6588):2015
Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM. High prevalence of occult hepatitis B virus infection in children born to HBsAg-positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J Hepatol 2012;57:515–521
Amponsah-Dacosta E, Lebelo RL, Rakgole JN, Selabe SG, Gededzha MP, Mayaphi SH, et al. Hepatitis B virus infection in post-vaccination South Africa: occult HBV infection and circulating surface gene variants. J Clin Virol. 2015;63:12–17
Li AY, Liu Z, Song Y, Xiao Y, Jiang J, Li L, et al. Reduction of the occurrence of occult HBV infection in infants by increasing the dose of hepatitis B vaccine: a large prospective cohort study. Emerg Microbes Infect 2020;9:1881–1891
Romano L, Carsetti R, Tozzi AE, Mele A, Zanetti AR. Chronic hepatitis B infection in adolescents vaccinated at birth: an alarm bell in favor of the need for a booster? Hepatology 2014;59:349
WHO. WHO guidelines approved by the guidelines review committee. In: Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015, Geneva
Acknowledgements
We sincerely thank all participants and their families in the Qidong Hepatitis B Intervention Study (QHBIS), and all staff who contributed to the initial intervention, and follow-up studies.
Funding
This work was supported by Key Research Projects for Precision Medicine (2017YFC0908103), Innovation Fund for Medical Sciences of Chinese Academy of Medical Sciences (CIFMS, 2019-I2M-2-004, Cohort study of the esophageal cancer and liver cancer; and 2016-I2M-1-007), State Key Projects Specialized on Infectious Diseases (2017ZX10201201-006), and National Natural Science Foundation Fund (81972628, 81571620).
Author information
Authors and Affiliations
Contributions
CQ designed and supervised the study, drafted and finalized the manuscript. RW, CL, TC, YW, CF, LL and FL performed the data collection and analysis. RW, CL and TC drafted the manuscript. FL provided the key reagents for HBV rcDNA detection and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Ruijun Wang, Chang Liu, Taoyang Chen, Yuting Wang, Chunsun Fan, Lingling Lu, Fengmin Lu, and Chunfeng Qu declare that they have no conflict of interest.
Ethics approval
The study protocol (NCC201709011) was approved by the Ethical Committees of the National Cancer Center/ Cancer Hospital, Chinese Academy of Medical Sciences (NCC/CH-CAMS). All procedures performed in studies involving human participants were in accordance with the ethical standards of 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
All participants provided written informed consent before any study procedures were provided.
Consent for publication
All authors of this manuscript have read and approved the final submitted version, and are aware that they are listed as an author on this paper.
Animal research
This article does not contain any studies with animals performed by any of the authors.
Clinical trials registration
The current study was based on a cross-sectional investigation of Qidong Hepatitis B Intervention Study (QHBIS). Original QHBIS was registered with Clinical Trials.gov number NCT00222664.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Liu, C., Chen, T. et al. Neonatal hepatitis B vaccination protects mature adults from occult virus infection. Hepatol Int 15, 328–337 (2021). https://doi.org/10.1007/s12072-021-10156-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10156-z