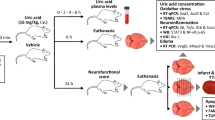
Abstract
Systemic inflammatory stimulus is a risk factor for the incidence of ischemic stroke and contributes to poorer clinical outcomes. Solute carrier 15A3 (SLC15A3) is a peptide/histidine transporter that is implicated in regulating inflammatory responses. However, whether SLC15A3 affects the progression of ischemic stroke associated with systemic inflammation is unclear. The transient middle cerebral artery occlusion (tMCAO) mice with LPS administration (LPS/tMCAO) were prepared as an in vivo model, and LPS-induced BV2 cells under oxygen–glucose deprivation (OGD) exposure were utilized as an in vitro model. We found that SLC15A3 was highly expressed in the ischemic penumbra of LPS/tMCAO mice, and its inhibition reduced infarct area, attenuated neurological deficit, recovered motor function, and mitigated apoptotic neurons. Knockdown of SLC15A3 suppressed the proinflammatory M1-type markers and promoted the levels of M2-associated genes. The in vitro results confirmed that SLC15A3 overexpression promoted microglia polarizing towards M1 subtypes, while SLC15A3 inhibition exerted an opposite effect. In addition, we demonstrated that the p65 signaling pathway and HIF1α were activated by LPS/OGD. Luciferase reporter assay showed that inhibiting p65 using its specific inhibitor BAY 11–7082 or silencing HIF1α using siRNAs reduced the transcriptional activity of SLC15A3 in LPS/OGD-induced BV2 cells. Results in NIH 3T3 cells also confirmed that p65 and HIF1α directly bound to the SLC15A3 promoter to activate SLC15A3 transcription. In conclusion, this work shows that SLC15A3, transcriptionally activated by p65 and HIF1α, contributes to poor outcomes in ischemic stroke associated with systemic inflammation by promoting microglial cells polarizing towards M1 types.







Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
References
Saini V, Guada L, Yavagal DR (2021) Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 97(20 Suppl 2):S6-s16
Schilling M et al (2005) Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 196(2):290–297
Hu X et al (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070
Wang J et al (2017) Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1-Dependent M1 Microglial Polarization. Stroke 48(8):2211–2221
Liu J et al (2019) Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization: a randomized controlled animal study. Crit Care 23(1):198
Fumagalli S et al (2013) CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 61(6):827–842
Palasik W et al (2005) Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. Eur Neurol 53(4):188–193
Emsley HC, Tyrrell PJ (2002) Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab 22(12):1399–1419
Dénes A, Ferenczi S, Kovács KJ (2011) Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation 8:164
McColl BW, Rothwell NJ, Allan SM (2007) Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci 27(16):4403–4412
Oppermann H et al (2019) The proton-coupled oligopeptide transporters PEPT2, PHT1 and PHT2 mediate the uptake of carnosine in glioblastoma cells. Amino Acids 51(7):999–1008
Nakamura N et al (2014) Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509(7499):240–244
Wang Y et al (2014) Expression and regulation of the proton-coupled oligopeptide transporter PhT2 by LPS in macrophages and mouse spleen. Mol Pharm 11(6):1880–1888
Song F et al (2018) Regulation and biological role of the peptide/histidine transporter SLC15A3 in Toll-like receptor-mediated inflammatory responses in macrophage. Cell Death Dis 9(7):770
Hu Y et al (2014) Divergent developmental expression and function of the proton-coupled oligopeptide transporters PepT2 and PhT1 in regional brain slices of mouse and rat. J Neurochem 129(6):955–965
Shvedova M et al (2021) Modified middle cerebral artery occlusion model provides detailed intraoperative cerebral blood flow registration and improves neurobehavioral evaluation. J Neurosci Methods 358:109179
Al Mamun A et al (2020) Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A 117(3):1742–1752
Al Mamun A et al (2018) Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur J Neurosci 47(2):140–149
Xue J et al (2019) Sphingomyelin Synthase 2 Inhibition Ameliorates Cerebral Ischemic Reperfusion Injury Through Reducing the Recruitment of Toll-Like Receptor 4 to Lipid Rafts. J Am Heart Assoc 8(22):e012885
Suenaga J et al (2015) White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 272:109–119
Daigneault M et al (2010) The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 5(1):e8668
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Meng H et al (2019) Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A 116(12):5558–5563
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Vatte S, Ugale R (2023) HIF-1, an important regulator in potential new therapeutic approaches to ischemic stroke. Neurochem Int 170:105605
Garcia JH, Liu KF, Ho KL (1995) Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke 26(4):636–42 (discussion 643)
Li Y, Zhang J (2021) Animal models of stroke. Animal Model Exp Med 4(3):204–219
Zhao N et al (2019) Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J Neuroinflammation 16(1):168
Bhattacharya R, Cabral F (2009) Molecular basis for class V beta-tubulin effects on microtubule assembly and paclitaxel resistance. J Biol Chem 284(19):13023–13032
Guérit D et al (2020) Primary myeloid cell proteomics and transcriptomics: importance of β-tubulin isotypes for osteoclast function. J Cell Sci 133:10
Lenglet S et al (2014) Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice. J Cereb Blood Flow Metab 34(5):802–812
Liang E et al (2021) COP9 Signalosome Subunit 3 Restricts Neuroinflammatory Responses During Cerebral Ischemia/Reperfusion Injury Through Stabilizing Suppressor of Cytokine Signaling 3 Protein. Neuropsychiatr Dis Treat 17:1217–1227
Liu S et al (2021) Differentially expressed genes induced by β-caryophyllene in a rat model of cerebral ischemia-reperfusion injury. Life Sci 273:119293
Sorce S et al (2010) Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol 160(2):311–321
Lalancette-Hébert M et al (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27(10):2596–2605
Won S, Lee JK, Stein DG (2015) Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain Behav Immun 49:267–279
Jackson-Cowan L et al (2021) Delayed Administration of Angiotensin Receptor (AT2R) Agonist C21 Improves Survival and Preserves Sensorimotor Outcomes in Female Diabetic Rats Post-Stroke through Modulation of Microglial Activation. Int J Mol Sci 22:3
Xu G et al (2024) The role and therapeutic potential of nuclear factor κB (NF-κB) in ischemic stroke. Biomed Pharmacother 171:116140
Baetz D, Shaw J, Kirshenbaum LA (2005) Nuclear factor-kappaB decoys suppress endotoxin-induced lung injury. Mol Pharmacol 67(4):977–979
Yao L et al (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10:23
Liu R et al (2019) Glycine Exhibits Neuroprotective Effects in Ischemic Stroke in Rats through the Inhibition of M1 Microglial Polarization via the NF-κB p65/Hif-1α Signaling Pathway. J Immunol 202(6):1704–1714
Liu C et al (2021) Genistein-3’-sodium sulfonate Attenuates Neuroinflammation in Stroke Rats by Down-Regulating Microglial M1 Polarization through α7nAChR-NF-κB Signaling Pathway. Int J Biol Sci 17(4):1088–1100
Liu H et al (2015) NOD2 is involved in the inflammatory response after cerebral ischemia-reperfusion injury and triggers NADPH oxidase 2-derived reactive oxygen species. Int J Biol Sci 11(5):525–535
Acknowledgements
Not applicable.
Funding
This study was supported by the Youth Fund Project of National Natural Science Foundation of China (82001226) and the Natural Science Foundation of Jilin Province (20210101357JC).
Author information
Authors and Affiliations
Contributions
SY and JHY designed research; SY, RZ, and JHY performed the research; SY, QG and LW analyzed data; SY and JHY wrote the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Ethics statement
The animal experiments were performed complied with the Guide for the Care and Use of Laboratory Animals, and the ethics were approved by the ethical committee of Jilin University (No. 202002031).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, S., Yang, J., Zhang, R. et al. SLC15A3 is transcriptionally regulated by HIF1α and p65 to worsen neuroinflammation in experimental ischemic stroke. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04191-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04191-8