Abstract
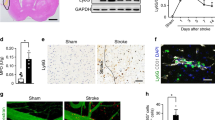
Cerebral venous thrombosis (CVT) is a neurovascular disease with recently increasing incidence. Aseptic inflammatory responses play an important role in the pathology of CVT. Recent studies report that neutrophil extracellular traps (NETs) are major triggers of thrombosis and inflammation in stroke, but their effect on brain injury in CVT requires further validation. In this study, two CVT animal models were used to simulate superior sagittal sinus thrombosis and cortical vein thrombosis. The effects of brain tissue infiltration of NETs and the molecular mechanisms associated with NET formation were deeply explored in combination with proteomics, histology, and serology. The results showed that the cortical vein thrombosis model could be combined with more severe blood–brain barrier (BBB) disruption and showed more severe cerebral hemorrhage. Decreased Sirtuin 1 (SIRT1) expression promotes high mobility group box 1 (HMGB1) acetylation, causing increased cytosolic translocation and extracellular release, and HMGB1 can promote NET formation and recruitment. In addition, corticocerebral accumulation of NETs contributes to BBB damage. This establishes a vicious cycle between BBB damage and NET accumulation. SIRT1 mediated-HMGB1 deacetylation may play a critical role in attenuating BBB damage following CVT. This study employed a combined validation using models of venous sinus thrombosis and cortical vein thrombosis to investigate the deacetylation role of SIRT1, aiming to offer new insights into the pathological mechanisms of brain injury following CVT.







Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed for the present study are available from the corresponding authors on reasonable request.
Abbreviations
- ADC :
-
Apparent diffusion coefficient
- BBB :
-
Blood-brain barrier
- CV :
-
Cortical vein
- CVT :
-
Cerebral venous thrombosis
- CitH3 :
-
Citrullinated histone H3
- COVID :
-
Coronavirus disease
- DAPI :
-
4′,6-Diamidino-2-phenylindole
- DMSO :
-
Dimethyl sulfoxide
- DNase :
-
Deoxyribonuclease
- DWI :
-
Diffusion-weighted imaging
- ELISA :
-
Enzyme‑linked immunosorbent assay
- EB :
-
Evans blue
- GO :
-
Gene Ontology
- HE :
-
Hematoxylin and eosin
- HMGB1 :
-
High mobility group box 1
- IL :
-
Interleukin
- IP :
-
Immunoprecipitation
- KEGG :
-
Kyoto Encyclopedia of Genes and Genomes
- MPO :
-
Myeloperoxidase
- NAD :
-
Nicotinamide adenosine dinucleotide
- NET :
-
Neutrophil extracellular trap
- OD :
-
Optical density
- PBS :
-
Phosphate-buffered saline
- SD :
-
Standard deviation
- SDS :
-
Sodium dodecyl sulfate
- SIRT1 :
-
Sirtuin 1
- SMA :
-
Smooth muscle actin
- SSS :
-
Superior sagittal sinus
- TUNEL :
-
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
- WB :
-
Western blotting
- ZO :
-
Zonula occludens
References
Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM et al (2011) Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(4):1158–1192. https://doi.org/10.1161/STR.0b013e31820a8364
Kowoll CM, Kaminski J, Weiß V, Bösel J, Dietrich W, Jüttler E, Flechsenhar J, Guenther A et al (2016) Severe cerebral venous and sinus thrombosis: clinical course, imaging correlates, and prognosis. Neurocrit Care 25(3):392–399. https://doi.org/10.1007/s12028-016-0256-8
Yang X, Wu F, Liu Y, Duan J, Meng R, Chen J, Li D, Fan Z et al (2019) Predictors of successful endovascular treatment in severe cerebral venous sinus thrombosis. Ann Clin Transl Neurol 6(4):755–761. https://doi.org/10.1002/acn3.749
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77(6):683–690. https://doi.org/10.1001/jamaneurol.2020.1127
Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, Aguiar De Sousa D, Sellner J et al (2021) Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol 28(10):3478–3490. https://doi.org/10.1111/ene.14727
Thakur KT, Tamborska A, Wood GK, McNeill E, Roh D, Akpan IJ, Miller EC, Bautista A et al (2021) Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: evaluation, management, and scientific questions. J Neurol Sci 427:117532. https://doi.org/10.1016/j.jns.2021.117532
Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, Ambler G et al (2021) Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med 385(18):1680–1689. https://doi.org/10.1056/NEJMoa2109908
Perry Richard J, Arina T, Bhagteshwar S, Brian C, Richard M, Peter A, Ming YJ, Liqun Z et al (2021) Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet (London, England) 398(10306):1147–1156. https://doi.org/10.1016/S0140-6736(21)01608-1
Ding J, Song B, Xie X, Li X, Chen Z, Wang Z, Pan L, Lan D et al (2022) Inflammation in cerebral venous thrombosis. Front Immunol 13:833490. https://doi.org/10.3389/fimmu.2022.833490
Guo Y, Zeng H, Gao C (2021) The role of neutrophil extracellular traps in central nervous system diseases and prospects for clinical application. Oxid Med Cell Longev 2021:9931742. https://doi.org/10.1155/2021/9931742
Manda-Handzlik A, Demkow U (2019) The brain entangled: the contribution of neutrophil extracellular traps to the diseases of the central nervous system. Cells 8(12):1477. https://doi.org/10.3390/cells8121477
Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H et al (2020) Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 11(1):2488. https://doi.org/10.1038/s41467-020-16191-y
Zeng H, Fu X, Cai J, Sun C, Yu M, Peng Y, Zhuang J, Chen J et al (2022) Neutrophil extracellular traps may be a potential target for treating early brain injury in subarachnoid hemorrhage. Transl Stroke Res 13(1):112–131. https://doi.org/10.1007/s12975-021-00909-1
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535. https://doi.org/10.1126/science.1092385
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW et al (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107(36):15880–15885. https://doi.org/10.1073/pnas.1005743107
Daniel C, Leppkes M, Muñoz LE, Schley G, Schett G, Herrmann M (2019) Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol 15(9):559–575. https://doi.org/10.1038/s41581-019-0163-2
Mu SW, Dang Y, Wang SS, Gu JJ (2018) The role of high mobility group box 1 protein in acute cerebrovascular diseases. Biomed Rep 9(3):191–197. https://doi.org/10.3892/br.2018.1127
Mu SW, Dang Y, Fan YC, Zhang H, Zhang JH, Wang W, Wang SS, Gu JJ (2019) Effect of HMGB1 and RAGE on brain injury and the protective mechanism of glycyrrhizin in intracranial-sinus occlusion followed by mechanical thrombectomy recanalization. Int J Mol Med 44(3):813–822. https://doi.org/10.3892/ijmm.2019.4248
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A et al (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22(20):5551–5560. https://doi.org/10.1093/emboj/cdg516
Stefania G, Cristina A, Denise F, Lotti Lavinia V, Torrisi Maria R, Bianchi Marco E, Anna R (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3(10):995–1001. https://doi.org/10.1093/embo-reports/kvf198
Kim SW, Lee JK (2020) Role of HMGB1 in the interplay between NETosis and thrombosis in ischemic stroke: a review. Cells 9(8):1794. https://doi.org/10.3390/cells9081794
Kim SW, Lee H, Lee HK, Kim ID, Lee JK (2019) Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun 7(1):94. https://doi.org/10.1186/s40478-019-0747-x
Rabadi MM, Xavier S, Vasko R, Kaur K, Goligorksy MS, Ratliff BB (2015) High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int 87(1):95–108. https://doi.org/10.1038/ki.2014.217
Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, Masuhr F, European Federation of Neurological Societies (2010) EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol 17(10):1229–1235. https://doi.org/10.1111/j.1468-1331.2010.03011.x
Mu S, Lin Y, Xu Y, Wei X, Zeng Z, Lin K, Zhu L, Liu Q et al (2022) A novel rat model for cerebral venous sinus thrombosis: verification of similarity to human disease via clinical analysis and experimental validation. J Transl Med 20(1):174. https://doi.org/10.1186/s12967-022-03374-y
Cai Q, Luo J, Ge S, Li Y, Cui W, Wu X, Li C, Wu Y et al (2020) The characteristics of brain injury following cerebral venous infarction induced by surgical interruption of the cortical bridging vein in mice. Brain Res 1739:146823. https://doi.org/10.1016/j.brainres.2020.146823
Bousser MG, Ferro JM (2007) Cerebral venous thrombosis: an update. Lancet Neurol 6(2):162–170. https://doi.org/10.1016/S1474-4422(07)70029-7
Schaller C, Nakase H, Kotani A, Nishioka T, Meyer B, Sakaki T (2002) Impairment of autoregulation following cortical venous occlusion in the rat. Neurol Res 24(2):210–214. https://doi.org/10.1179/016164102101199620
Wei S, Gao Y, Dai X, Fu W, Cai S, Fang H, Zeng Z, Chen Z (2019) SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol 316(1):F20–F31. https://doi.org/10.1152/ajprenal.00119.2018
Gao X, Hao S, Yan H, Ding W, Li K, Li J (2015) Neutrophil extracellular traps contribute to the intestine damage in endotoxemic rats. J Surg Res 195(1):211–218. https://doi.org/10.1016/j.jss.2014.12.019
Hopp S, Nolte MW, Stetter C, Kleinschnitz C, Sirén AL, Albert-Weissenberger C (2017) Alleviation of secondary brain injury, posttraumatic inflammation, and brain edema formation by inhibition of factor XIIa. J Neuroinflammation 14(1):39. https://doi.org/10.1186/s12974-017-0815-8
Jiqin Z, Fei S, Xiaojing Z, Hua J, Xiuqi W, Biao W, Min Z, Mi T et al (2017) EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol Cancer 16(1):127. https://doi.org/10.1186/s12943-017-0704-x
Iachettini S, Ciccarone F, Maresca C, D’Angelo C, Petti E, Di Vito S, Ciriolo MR, Zizza P et al (2022) The telomeric protein TERF2/TRF2 impairs HMGB1-driven autophagy. Autophagy 19(5):1479–1490. https://doi.org/10.1080/15548627.2022.2138687
Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, Peng C, Zhou T et al (2021) Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy 17(5):1157–1169. https://doi.org/10.1080/15548627.2020.1752471
Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L et al (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184(2):205–213. https://doi.org/10.1083/jcb.200806072
Brinkmann V (2018) Neutrophil extracellular traps in the second decade. J Innate Immun 10(5–6):414–421. https://doi.org/10.1159/000489829
An Z, Li J, Yu J, Wang X, Gao H, Zhang W, Wei Z, Zhang J et al (2019) Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 18(21):2928–2938. https://doi.org/10.1080/15384101.2019.1662678
Kazmierski R, Michalak S, Wencel-Warot A, Nowinski WL (2012) Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology 79(16):1677–1685. https://doi.org/10.1212/WNL.0b013e31826e9a83
Zheng S, Wang C, Lin L, Mu S, Liu H, Hu X, Chen X, Wang S (2022) TNF-α impairs pericyte-mediated cerebral microcirculation via the NF-κB/iNOS axis after experimental traumatic brain injury. J Neurotrauma 40(3–4):349–364. https://doi.org/10.1089/neu.2022.0016
Xie F, Tan Q, Yu A, Guo P, Wang L, Zeng Z, Liang L, Xian J et al (2021) The role of cell-free DNA in fibrinolysis for intraventricular hemorrhage. J Neurosurg 135(4):1105–1112. https://doi.org/10.3171/2020.7.JNS201429
Peña-Martínez C, Durán-Laforet V, García-Culebras A, Cuartero MI, Moro MÁ, Lizasoain I (2022) Neutrophil extracellular trap targeting protects against ischemic damage after fibrin-rich thrombotic stroke despite non-reperfusion. Front Immunol 13:790002. https://doi.org/10.3389/fimmu.2022.790002
Wang R, Zhu Y, Liu Z, Chang L, Bai X, Kang L, Cao Y, Yang X et al (2021) Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood 138(1):91–103. https://doi.org/10.1182/blood.2020008913
Juana V, Aída L, Teresa SM, María LA, Tembl José I, Salom Juan B, Candela N, Antonio M (2017) Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 117(10):1919–1929. https://doi.org/10.1160/TH17-02-0130
Frederik D, Irina P, Rustad John L, Cody Mark J, de Araujo CV, Chieko H, Alexander Matthew D, Ramesh G et al (2022) Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest 132(10):e154225. https://doi.org/10.1172/JCI154225
Puy L, Corseaux D, Perbet R, Deramecourt V, Cordonnier C, Bérézowski V (2021) Neutrophil extracellular traps (NETs) infiltrate haematoma and surrounding brain tissue after intracerebral haemorrhage: a post-mortem study. Neuropathol Appl Neurobiol 47(6):867–877. https://doi.org/10.1111/nan.12733
Enzmann G, Mysiorek C, Gorina R, Cheng YJ, Ghavampour S, Hannocks MJ, Prinz V, Dirnagl U et al (2013) The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol 125(3):395–412. https://doi.org/10.1007/s00401-012-1076-3
Isabel P, Francesc M, Maura F, Ellen G, Jordi P, Carles J, Xabier U, Angel C et al (2015) Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129(2):239–257. https://doi.org/10.1007/s00401-014-1381-0
Urbonaviciute V, Voll RE (2011) High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J Intern Med 270(4):309–318. https://doi.org/10.1111/j.1365-2796.2011.02432.x
Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J et al (2015) Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 62(2):600–614. https://doi.org/10.1002/hep.27841
Wu H, Li R, Pei LG, Wei ZH, Kang LN, Wang L, Xie J, Xu B (2018) Emerging role of high mobility group box-1 in thrombosis-related diseases. Cell Physiol Biochem 47(4):1319–1337. https://doi.org/10.1159/000490818
Zhou Y, Zhang F, Ding J (2022) As a modulator, multitasking roles of SIRT1 in respiratory diseases. Immune Netw 22(3):e21. https://doi.org/10.4110/in.2022.22.e21
Zainal N, Chang CP, Cheng YL, Wu YW, Anderson R, Wan SW, Chen CL, Ho TS et al (2017) Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci Rep 7:42998. https://doi.org/10.1038/srep42998
Lu CL, Liao MT, Hou YC, Fang YW, Zheng CM, Liu WC, Chao CT, Lu KC et al (2020) Sirtuin-1 and its relevance in vascular calcification. Int J Mol Sci 21(5):1593. https://doi.org/10.3390/ijms21051593
Li C, Xing Y, Zhang Y, Hua Y, Hu J, Bai Y (2022) Neutrophil extracellular traps exacerbate ischemic brain damage. Mol Neurobiol 59(1):643–656. https://doi.org/10.1007/s12035-021-02635-z
Rashad S, Niizuma K, Sato-Maeda M, Fujimura M, Mansour A, Endo H, Ikawa S, Tominaga T (2018) Early BBB breakdown and subacute inflammasome activation and pyroptosis as a result of cerebral venous thrombosis. Brain Res 1699:54–68. https://doi.org/10.1016/j.brainres.2018.06.029
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Allen C, Thornton P, Denes A, McColl BW, Pierozynski A, Monestier M, Pinteaux E, Rothwell NJ et al (2012) Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol 189(1):381–392. https://doi.org/10.4049/jimmunol.1200409
Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W et al (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187(1):538–552. https://doi.org/10.4049/jimmunol.1100450
Meegan JE, Yang X, Beard RS Jr, Jannaway M, Chatterjee V, Taylor-Clark TE, Yuan SY (2018) Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun 503(3):1498–1502. https://doi.org/10.1016/j.bbrc.2018.07.069
Smyth L, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, O’Carroll SJ, Mee EW et al (2018) Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation 15(1):138. https://doi.org/10.1186/s12974-018-1167-8
Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209(6):1219–1234. https://doi.org/10.1084/jem.20111622
Liu YW, Zhang J, Bi W, Zhou M, Li J, Xiong T, Yang N, Zhao L et al (2022) Histones of neutrophil extracellular traps induce CD11b expression in brain pericytes via dectin-1 after traumatic brain injury. Neurosci Bull 38(10):1199–1214. https://doi.org/10.1007/s12264-022-00902-0
Chen CC, Chen X, Li TC, Lin HL, Chu YT, Lee HC, Cheng YK, Chen DC et al (2017) PG2 for patients with acute spontaneous intracerebral hemorrhage: a double-blind, randomized, placebo-controlled study. Sci Rep 7:45628. https://doi.org/10.1038/srep45628
Jin J, Zhao X, Li W, Wang F, Tian J, Wang N, Gao X, Zhang J et al (2022) Neutrophil extracellular traps: a novel therapeutic target for intracranial hemorrhage. Thromb Res 219:1–13. https://doi.org/10.1016/j.thromres.2022.08.024
Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Ben Maacha M, Blanc R et al (2018) Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 49(3):754–757. https://doi.org/10.1161/STROKEAHA.117.019896
Katrin WA, Luise E, Jan R (2022) Platelets in the NETworks interweaving inflammation and thrombosis. Front Immunol 13:953129. https://doi.org/10.3389/fimmu.2022.953129
Dejun X, Lingbin L, Yongju Z, Li Y, Jianyong C, Rongmao H, Zelin Z, Qingwang L (2020) Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J Pineal Res 69(4):e12690. https://doi.org/10.1111/jpi.12690
Wang H, Guan Y, Karamercan MA, Ye L, Bhatti T, Becker LB, Baur JA, Sims CA (2015) Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock 44(2):173–180. https://doi.org/10.1097/SHK.0000000000000390
Zeng Z, Chen Z, Xu S, Song R, Yang H, Zhao KS (2015) Polydatin alleviates small intestine injury during hemorrhagic shock as a SIRT1 activator. Oxid Med Cell Longev 2015:965961. https://doi.org/10.1155/2015/965961
Acknowledgements
Sincere appreciation is given to the teachers and our colleagues from Fuzong Clinical Medical College, who participated in this study with great cooperation.
Funding
This study was supported by Joint Funds for the Innovation of Science and Technology, Fujian province (no. 2019Y9045); Joint Funds for the Innovation of Science and Technology, Fujian province (no. 2019Y9042); China Postdoctoral Science Foundation (no. 2021M693955); and Startup Fund for Scientific Research at Fujian Medical University (no. 2020QH2040).
Author information
Authors and Affiliations
Contributions
S.W., Y.X., and S.M. were responsible for the study concept and design. S.M., L.L., and L.X. performed animal model operation. S.M., Z.L., L.L., L.C., Y.L., D.Y., K.L., and Y.Y. performed the proteomic, histological, and serological experiments. S.M., D.W., F.Y., and L.W. were responsible for the data analyses. S.W. and Z.L. drafted the manuscript. S.W. and Y.X. critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This experiment was approved by the Animal Care and Use Committee of Fujian Medical University (2020–051) and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, S., Li, Z., Lin, L. et al. SIRT1-Mediated HMGB1 Deacetylation Suppresses Neutrophil Extracellular Traps Related to Blood–Brain Barrier Impairment After Cerebral Venous Thrombosis. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03959-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03959-2