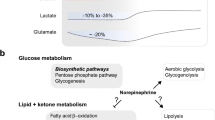
Abstract
Lactate is not only the energy substrate of neural cells, but also an important signal molecule in brain. In modern societies, disturbed circadian rhythms pose a global challenge. Therefore, exploring the influence of circadian period on lactate and its metabolic kinetics is essential for the advancement of neuroscientific research. In the present study, the different groups of mice (L: 8:00 a.m.; D: 20:00 p.m.; SD: 20:00 p.m. with 12 h acute sleep deprivation) were infused with [3-13C] lactate through the lateral tail vein for a duration of 2 min. After 30-min lactate metabolism, the animals were euthanized and the tissues of brain and liver were obtained and extracted, and then, the [1H-13C] NMR technology was employed to investigate the kinetic information of lactate metabolism in different brain regions and liver to detect the enrichment of various metabolic kinetic information. Results revealed the fluctuating lactate concentrations in the brain throughout the day, with lower levels during light periods and higher levels during dark periods. Most metabolites displayed strong sensitivity to circadian rhythm, exhibiting significant day-night variations. Conversely, only a few metabolites showed changes after acute sleep deprivation, primarily in the temporal brain region. Interestingly, in contrast to brain lactate metabolism, liver lactate metabolism exhibited a significant increase following acute sleep deprivation. This study explored the kinetics of lactate metabolism, hinted at potential clinical implications for disorders involving circadian rhythm disturbances, and providing a new research basis for clinical exploration of brain and liver lactate metabolism.







Similar content being viewed by others
Data Availability
All raw data and materials during the current study are available from the corresponding author upon reasonable request.
References
Seaquist ER, Öz G (2013) Diabetes: does lactate sustain brain metabolism during hypoglycaemia? Nat Rev Endocrinol 9:386–387. https://doi.org/10.1038/nrendo.2013.104
Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L (2003) Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A 100:4879–4884. https://doi.org/10.1073/pnas.0831078100
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91:10625–10629. https://doi.org/10.1073/pnas.91.22.10625
Poggiogalle E, Jamshed H, Peterson CM (2018) Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 84:11–27. https://doi.org/10.1016/j.metabol.2017.11.017
Vasey C, McBride J, Penta K (2021) Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13:3480. https://doi.org/10.3390/nu13103480
Bajaj P, Kaur G (2022) Acute sleep deprivation-induced anxiety and disruption of hypothalamic cell survival and plasticity: a mechanistic study of protection by butanol extract of Tinospora cordifolia. Neurochem Res 47:1692–1706. https://doi.org/10.1007/s11064-022-03562-8
Li Z, Chai W (2019) Mucin O-glycan microarrays. Curr Opin Struct Biol 56:187–197. https://doi.org/10.1016/j.sbi.2019.03.032
Guo M, Wu Y, Zheng D, Chen L, Xiong B, Wu J, Li K, Wang L et al (2022) Preoperative acute sleep deprivation causes postoperative pain hypersensitivity and abnormal cerebral function. Neurosci Bull 38:1491–1507. https://doi.org/10.1007/s12264-022-00955-1
Ma Y, Liang L, Zheng F, Shi L, Zhong B, Xie W (2020) Association between sleep duration and cognitive decline. JAMA Netw Open 3:e2013573. https://doi.org/10.1001/jamanetworkopen.2020.13573
Wu H, Dunnett S, Ho YS, Chang RC (2019) The role of sleep deprivation and circadian rhythm disruption as risk factors of Alzheimer’s disease. Front Neuroendocrinol 54:100764. https://doi.org/10.1016/j.yfrne.2019.100764
Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, Kupfer DJ, Moore RY (2004) Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep 27:1245–1254. https://doi.org/10.1093/sleep/27.7.1245
Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI et al (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22:1523–1531. https://doi.org/10.1523/jneurosci.22-05-01523.2002
Feneberg R, Sparber M, Veldhuis JD, Mehls O, Ritz E, Schaefer F (1999) Synchronous fluctuations of blood insulin and lactate concentrations in humans. J Clin Endocrinol Metab 84:220–227. https://doi.org/10.1210/jcem.84.1.5377
Wallace NK, Pollard F, Savenkova M, Karatsoreos IN (2020) Effect of aging on daily rhythms of lactate metabolism in the medial prefrontal cortex of male mice. Neuroscience 448:300–310. https://doi.org/10.1016/j.neuroscience.2020.07.032
Koopmans SJ, van der Meulen J, Dekker R, Corbijn H, Mroz Z (2005) Diurnal rhythms in plasma cortisol, insulin, glucose, lactate and urea in pigs fed identical meals at 12-hourly intervals. Physiol Behav 84:497–503. https://doi.org/10.1016/j.physbeh.2005.01.017
Robinson JL, Foustock S, Chanez M, Bois-Joyeux B, Peret J (1981) Circadian variation of liver metabolites and amino acids in rats adapted to a high protein, carbohydrate-free diet. J Nutr 111:1711–1720. https://doi.org/10.1093/jn/111.10.1711
Ahlersová E, Ahlers I, Toropila M, Smajda B, Datelinka I (1981) Circadian rhythm of the lactate and pyruvate concentration in rat liver and blood. Physiol Bohemoslov 30:213–220
Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, Rossi MJ, Elmsaouri S et al (2020) Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron 108:937-952.e7. https://doi.org/10.1016/j.neuron.2020.09.002
Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M (2017) Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37:2112–2124. https://doi.org/10.1177/0271678x16661202
Killgore WD (2010) Effects of sleep deprivation on cognition. Prog Brain Res 185:105–129. https://doi.org/10.1016/b978-0-444-53702-7.00007-5
Zimmet P, Alberti K, Stern N, Bilu C, El-Osta A, Einat H, Kronfeld-Schor N (2019) The circadian syndrome: is the metabolic syndrome and much more! J Intern Med 286:181–191. https://doi.org/10.1111/joim.12924
Matsubara Y, Kiyohara H, Teratani T, Mikami Y, Kanai T (2022) Organ and brain crosstalk: the liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology 205:108915. https://doi.org/10.1016/j.neuropharm.2021.108915
Shram N, Netchiporouk L, Cespuglio R (2002) Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci 16:461–466. https://doi.org/10.1046/j.1460-9568.2002.02081.x
Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Johnson DA, Harmon HP, Gabbert S et al (2012) Lactate as a biomarker for sleep. Sleep 35:1209–1222. https://doi.org/10.5665/sleep.2072
de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL (2003) In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed 16:339–357. https://doi.org/10.1002/nbm.847
Guo M, Fang Y, Zhu J, Chen C, Zhang Z, Tian X, Xiang H, Manyande A et al (2021) Investigation of metabolic kinetics in different brain regions of awake rats using the [(1)H-(13)C]-NMR technique. J Pharm Biomed Anal 204:114240. https://doi.org/10.1016/j.jpba.2021.114240
Hassel B, Bråthe A (2000) Cerebral metabolism of lactate in vivo: evidence for neuronal pyruvate carboxylation. J Cereb Blood Flow Metab 20:327–336. https://doi.org/10.1097/00004647-200002000-00014
Liu T, Li Z, He J, Yang N, Han D, Li Y, Tian X, Liu H et al (2020) Regional metabolic patterns of abnormal postoperative behavioral performance in aged mice assessed by (1)H-NMR dynamic mapping method. Neurosci Bull 36:25–38. https://doi.org/10.1007/s12264-019-00414-4
Zhu J, Chen C, Li Z, Liu X, He J, Zhao Z, He M, Nie B et al (2023) Overexpression of Sirt6 ameliorates sleep deprivation induced-cognitive impairment by modulating glutamatergic neuron function. Neural Regen Res 18:2449–2458. https://doi.org/10.4103/1673-5374.371370
Wu L, Niu Z, Hu X, Liu H, Li S, Chen L, Zheng D, Liu Z et al (2020) Regional cerebral metabolic levels and turnover in awake rats after acute or chronic spinal cord injury. Faseb j 34:10547–10559. https://doi.org/10.1096/fj.202000447R
Liu Y, Cheng J, Liu H, Deng Y, Wang J, Xu F (2017) NMRSpec: an integrated software package for processing and analyzing one dimensional nuclear magnetic resonance spectra. Chemom Intell Lab Syst 162:142–148. https://doi.org/10.1016/j.chemolab.2017.01.005
Takado Y, Cheng T, Bastiaansen JAM, Yoshihara HAI, Lanz B, Mishkovsky M, Lengacher S, Comment A (2018) Hyperpolarized (13)C magnetic resonance spectroscopy reveals the rate-limiting role of the blood-brain barrier in the cerebral uptake and metabolism of l-lactate in vivo. ACS Chem Neurosci 9:2554–2562. https://doi.org/10.1021/acschemneuro.8b00066
Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL (2002) [2,4–13 C2 ]-beta-Hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22:890–898. https://doi.org/10.1097/00004647-200207000-00014
Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL (2010) The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 30:13983–13991. https://doi.org/10.1523/jneurosci.2040-10.2010
Kamel KS, Oh MS, Halperin ML (2020) L-lactic acidosis: pathophysiology, classification, and causes; emphasis on biochemical and metabolic basis. Kidney Int 97:75–88. https://doi.org/10.1016/j.kint.2019.08.023
Isobe Y, Hida H, Nishino H (2011) Circadian rhythm of metabolic oscillation in suprachiasmatic nucleus depends on the mitochondrial oxidation state, reflected by cytochrome C oxidase and lactate dehydrogenase. J Neurosci Res 89:929–935. https://doi.org/10.1002/jnr.22609
Newman GC, Hospod FE, Patlak CS, Moore RY (1992) Analysis of in vitro glucose utilization in a circadian pacemaker model. J Neurosci 12:2015–2021. https://doi.org/10.1523/jneurosci.12-06-02015.1992
Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, Cui N, Middleton B et al (2014) Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A 111:10761–10766. https://doi.org/10.1073/pnas.1402663111
Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, Franken P, Tafti M (2012) Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci 32:12506–12517. https://doi.org/10.1523/jneurosci.2306-12.2012
Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, Nissim I (2006) Short-term fasting, seizure control and brain amino acid metabolism. Neurochem Int 48:650–656. https://doi.org/10.1016/j.neuint.2006.01.008
Tanay VA, Parent MB, Wong JT, Paslawski T, Martin IL, Baker GB (2001) Effects of the antidepressant/antipanic drug phenelzine on alanine and alanine transaminase in rat brain. Cell Mol Neurobiol 21:325–339. https://doi.org/10.1023/a:1012697904299
Das A, Gauthier-Coles G, Bröer S, Rae CD (2022) Impact of inhibition of glutamine and alanine transport on cerebellar glial and neuronal metabolism. Biomolecules 12:1189. https://doi.org/10.3390/biom12091189
Morikawa A, Hamase K, Miyoshi Y, Koyanagi S, Ohdo S, Zaitsu K (2008) Circadian changes of D-alanine and related compounds in rats and the effect of restricted feeding on their amounts. J Chromatogr B Analyt Technol Biomed Life Sci 875:168–173. https://doi.org/10.1016/j.jchromb.2008.04.004
Sivaperumal R, Subash S, Subramanian P (2007) Influences of aspartate on circadian patterns of lipid peroxidation products and antioxidants in Wistar rats. Singapore Med J 48:1033–1038
Honma S, Katsuno Y, Shinohara K, Abe H, Honma K (1996) Circadian rhythm and response to light of extracellular glutamate and aspartate in rat suprachiasmatic nucleus. Am J Physiol 271:R579–R585. https://doi.org/10.1152/ajpregu.1996.271.3.R579
Morzorati SL, McBride WJ, Frederickson RC (1981) Excitatory effect of L-aspartate and L-glutamate on Purkinje cells in rat cerebellum. Brain Res Bull 7:445–447. https://doi.org/10.1016/0361-9230(81)90045-9
Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 95:316–321. https://doi.org/10.1073/pnas.95.1.316
Andersen JV, Schousboe A, Verkhratsky A (2022) Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: integration of the glutamate/GABA-glutamine cycle. Prog Neurobiol 217:102331. https://doi.org/10.1016/j.pneurobio.2022.102331
Özakman S, Gören MZ, Nurten A, Tekin N, Kalaycı R, Enginar N (2021) Effects of tamoxifen and glutamate and glutamine levels in brain regions in repeated sleep deprivation-induced mania model in mice. Naunyn Schmiedebergs Arch Pharmacol 394:619–629. https://doi.org/10.1007/s00210-020-02001-1
Bettendorff L, Sallanon-Moulin M, Touret M, Wins P, Margineanu I, Schoffeniels E (1996) Paradoxical sleep deprivation increases the content of glutamate and glutamine in rat cerebral cortex. Sleep 19:65–71. https://doi.org/10.1093/sleep/19.1.65
Mohammed HS, Aboul Ezz HS, Khadrawy YA, Noor NA (2011) Neurochemical and electrophysiological changes induced by paradoxical sleep deprivation in rats. Behav Brain Res 225:39–46. https://doi.org/10.1016/j.bbr.2011.06.018
Xie F, Li X, Bao M, Shi R, Yue Y, Guan Y, Wang Y (2015) Anesthetic propofol normalized the increased release of glutamate and γ-amino butyric acid in hippocampus after paradoxical sleep deprivation in rats. Neurol Res 37:1102–1107. https://doi.org/10.1080/01616412.2015.1114231
Mohammed HS, Khadrawy YA (2021) Electrophysiological and neurochemical evaluation of the adverse effects of REM sleep deprivation and epileptic seizures on rat’s brain. Life Sci 273:119303. https://doi.org/10.1016/j.lfs.2021.119303
Foppen E, Tan AA, Ackermans MT, Fliers E and Kalsbeek A (2016) Suprachiasmatic nucleus neuropeptides and their control of endogenous glucose production. J Neuroendocrinol 28(4). https://doi.org/10.1111/jne.12365
Archer SN, Oster H (2015) How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res 24:476–493. https://doi.org/10.1111/jsr.12307
Geisler CE, Ghimire S, Hepler C, Miller KE, Bruggink SM, Kentch KP, Higgins MR, Banek CT et al (2021) Hepatocyte membrane potential regulates serum insulin and insulin sensitivity by altering hepatic GABA release. Cell Rep 35:109298. https://doi.org/10.1016/j.celrep.2021.109298
Periasamy S, Hsu DZ, Fu YH, Liu MY (2015) Sleep deprivation-induced multi-organ injury: role of oxidative stress and inflammation. Excli J 14:672–83. https://doi.org/10.17179/excli2015-245
Shigiyama F, Kumashiro N, Tsuneoka Y, Igarashi H, Yoshikawa F, Kakehi S, Funato H, Hirose T (2018) Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. Am J Physiol Endocrinol Metab 315:E848-e858. https://doi.org/10.1152/ajpendo.00072.2018
Wang C, Li L, Yang C, Zhang Z, Li X, Wang Y, Lv X, Qi X, Song G (2022) One night of sleep deprivation induces release of small extracellular vesicles into circulation and promotes platelet activation by small EVs. J Cell Mol Med 26:5033–5043. https://doi.org/10.1111/jcmm.17528
Butterworth RF (2013) The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol 10:522–528. https://doi.org/10.1038/nrgastro.2013.99
Lezi E, Lu J, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127:91–100. https://doi.org/10.1111/jnc.12394
Yan L, Wei JA, Yang F, Wang M, Wang S, Cheng T, Liu X, Jia Y et al (2022) Physical exercise prevented stress-induced anxiety via improving brain RNA methylation. Adv Sci (Weinh) 9:e2105731. https://doi.org/10.1002/advs.202105731
Hadjihambi A, Konstantinou C, Klohs J, Monsorno K, Le Guennec A, Donnelly C, Cox IJ, Kusumbe A et al (2023) Partial MCT1 invalidation protects against diet-induced non-alcoholic fatty liver disease and the associated brain dysfunction. J Hepatol 78:180–190. https://doi.org/10.1016/j.jhep.2022.08.008
Alvord VM, Kantra EJ, Pendergast JS (2022) Estrogens and the circadian system. Semin Cell Dev Biol 126:56–65. https://doi.org/10.1016/j.semcdb.2021.04.010
Funding
This work was supported by grants from the National Natural Science Foundation of China (82172160, 81970722, 82001119, 31970973, 82270861, 82271430, and 32271148).
Author information
Authors and Affiliations
Contributions
Lili Chen, Kefan Wu, Jie Wang, and Zhongyuan Xia designed the study. Lili Chen, Kefan Wu, Jiabao Hou, Lian Liu, and Yuan Zhang performed the experiments. Lili Chen, Kefan Wu, and Jingang He contributed to the data. Lili Chen and Kefan Wu wrote the manuscript. Jie Wang and Zhongyuan Xia edited the article. The content of this manuscript has been reviewed, read, and agreed upon by all the designated authors.
Corresponding authors
Ethics declarations
Ethics Approval
The animal study was reviewed and approved by the Animal Research Committee of the Renmin Hospital of Wuhan University. (Ethics approval number: WDRM20220802A).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Wu, K., He, J. et al. Circadian Regulation of the Lactate Metabolic Kinetics in Mice Using the [1H-13C]-NMR Technique. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03927-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03927-w