Abstract
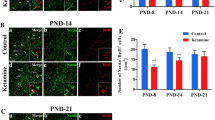
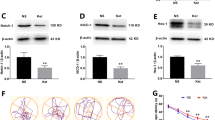
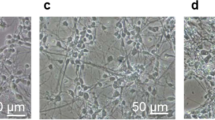
The mechanism of ketamine-induced neurotoxicity development remains elusive. Mitochondrial fusion/fission dynamics play a critical role in regulating neurogenesis. Therefore, this study was aimed to evaluate whether mitochondrial dynamics were involved in ketamine-induced impairment of neurogenesis in neonatal rats and long-term synaptic plasticity dysfunction. In the in vivo study, postnatal day 7 (PND-7) rats received intraperitoneal (i.p.) injection of 40 mg/kg ketamine for four consecutive times at 1 h intervals. The present findings revealed that ketamine induced mitochondrial fusion dysfunction in hippocampal neural stem cells (NSCs) by downregulating Mitofusin 2 (Mfn2) expression. In the in vitro study, ketamine treatment at 100 μM for 6 h significantly decreased the Mfn2 expression, and increased ROS generation, decreased mitochondrial membrane potential and ATP levels in cultured hippocampal NSCs. For the interventional study, lentivirus (LV) overexpressing Mfn2 (LV-Mfn2) or control LV vehicle was microinjected into the hippocampal dentate gyrus (DG) 4 days before ketamine administration. Targeted Mfn2 overexpression in the DG region could restore mitochondrial fusion in NSCs and reverse the inhibitory effect of ketamine on NSC proliferation and its faciliatory effect on neuronal differentiation. In addition, synaptic plasticity was evaluated by transmission electron microscopy, Golgi-Cox staining and long-term potentiation (LTP) recordings at 24 h after the end of the behavioral test. Preconditioning with LV-Mfn2 improved long-term cognitive dysfunction after repeated neonatal ketamine exposure by reversing the inhibitory effect of ketamine on synaptic plasticity in the hippocampal DG. The present findings demonstrated that Mfn2-mediated mitochondrial fusion dysfunction plays a critical role in the impairment of long-term neurocognitive function and synaptic plasticity caused by repeated neonatal ketamine exposure by interfering with hippocampal neurogenesis. Thus, Mfn2 might be a novel therapeutic target for the prevention of the developmental neurotoxicity of ketamine.











Similar content being viewed by others
Data Availability
For further inquiries on the original data of this study, the corresponding authors can be contacted directly.
Abbreviations
- BGS:
-
Brain growth spurt
- BrdU:
-
Bromodeoxyuridine
- CA:
-
Cornu ammonis
- DCX:
-
Doublecortin
- DG:
-
Dentate gyrus
- Drp1:
-
Fission-related protein 1
- fEPSP:
-
Field excitatory postsynaptic potential
- GCL:
-
Granular cell layer
- LTP:
-
Long-term potentiation
- Mfn:
-
Mitofusins
- MWM:
-
Morris water maze
- NMDA:
-
N-methyl-D-aspartate
- NSCs:
-
Neural stem cells
- OPA1:
-
Optic atrophy 1
- PND:
-
Postnatal day
- PSD:
-
Postsynaptic density
- ROS:
-
Reactive oxygen species
- SD:
-
Sprague‒Dawley
- TBS:
-
Theta burst stimulation
- TEM:
-
Transmission Electron Microscopy
References
Jevtovic-Todorovic V et al (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882
Davidson AJ et al (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387(10015):239–250
McCann ME et al (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393(10172):664–677
Lee JJ, Sun LS, Levy RJ (2019) Report on the Sixth Pediatric Anesthesia Neurodevelopmental Assessment (PANDA) Symposium, “Anesthesia and Neurodevelopment in Children.” J Neurosurg Anesthesiol 31(1):103–107
Warner DO et al (2018) Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 129(1):89–105
Zhang Z, Bai H, Ma X, Shen M, Li R, Qiu D, Li S, Gao L (2021) Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats. J Neuroinflammation 18(1):239
Cabrera OH, Useinovic N, Maksimovic S, Near M, Quillinan N, Todorovic SM, Jevtovic-Todorovic V (2022) Neonatal ketamine exposure impairs infrapyramidal bundle pruning and causes lasting increase in excitatory synaptic transmission in hippocampal CA3 neurons. Neurobiol Dis 175:105923
Dong C, Rovnaghi CR, Anand KJ (2016) Ketamine exposure during embryogenesis inhibits cellular proliferation in rat fetal cortical neurogenic regions. Acta Anaesthesiol Scand 60(5):579–587
Ito H, Uchida T, Makita K (2015) Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLoS ONE 10(5):e0128445
Fuentealba LC et al (2015) Embryonic origin of postnatal neural stem cells. Cell 161(7):1644–1655
Bandeira F, Lent R, Herculano-Houzel S (2009) Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A 106(33):14108–14113
Bartley J et al (2005) BrdU-positive cells in the neonatal mouse hippocampus following hypoxic-ischemic brain injury. BMC Neurosci 6:15
Belnoue L et al (2013) Prenatal stress inhibits hippocampal neurogenesis but spares olfactory bulb neurogenesis. PLoS ONE 8(8):e72972
Porzionato A et al (2015) Effects of postnatal hyperoxia exposure on the rat dentate gyrus and subventricular zone. Brain Struct Funct 220(1):229–247
Huang H et al (2016) Ketamine affects the neurogenesis of the hippocampal dentate gyrus in 7-day-old rats. Neurotox Res 30(2):185–198
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11(12):872–884
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99
Khacho M et al (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19(2):232–247
Iwata R, Casimir P, Vanderhaeghen P (2020) Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science 369(6505):858–862
Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE (1982) Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg 61(2):87–92
Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ (2013) Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116(4):869–880
Huang H et al (2021) Neonatal anesthesia by ketamine in neonatal rats inhibits the proliferation and differentiation of hippocampal neural stem cells and decreases neurocognitive function in adulthood via inhibition of the Notch1 signaling pathway. Mol Neurobiol 58(12):6272–6289
Khazipov R et al (2015) Atlas of the postnatal rat brain in stereotaxic coordinates. Front Neuroanat 9:161
Wan J et al (2021) Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood. Brain Res Bull 169:63–72
Schmitz A et al (2018) Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics-A prospective randomized double-blinded study. Paediatr Anaesth 28(3):264–274
Andropoulos DB, Greene MF (2017) Anesthesia and developing brains - implications of the FDA warning. N Engl J Med 376(10):905–907
Bayer SA et al (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14(1):83–144
Huang J et al (2016) Propofol administration during early postnatal life suppresses hippocampal neurogenesis. Mol Neurobiol 53(2):1031–1044
Stratmann G et al (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110(4):834–848
Fang F, Xue Z, Cang J (2012) Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci Bull 28(5):499–508
Tresse E et al (2021) IFN-β rescues neurodegeneration by regulating mitochondrial fission via STAT5, PGAM5, and Drp1. Embo J 40(11):e106868
Dong L et al (2020) Promotion of mitochondrial fusion protects against developmental PBDE-47 neurotoxicity by restoring mitochondrial homeostasis and suppressing excessive apoptosis. Theranostics 10(3):1245–1261
Boscolo A et al (2013) Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology 118(5):1086–1097
Amrock LG et al (2015) Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology 122(1):87–95
Liu J et al (2022) Sevoflurane induced neurotoxicity in neonatal mice links to a GSK3beta/Drp1-dependent mitochondrial fission and apoptosis. Free Radic Biol Med 181:72–81
Ozsoy S et al (2021) Cannibalized erythroblasts accelerate developmental neurogenesis by regulating mitochondrial dynamics. Cell Rep 35(1):108942
Channakkar AS et al (2020) MiRNA-137-mediated modulation of mitochondrial dynamics regulates human neural stem cell fate. Stem Cells 38(5):683–697
Iwata R, Vanderhaeghen P (2021) Regulatory roles of mitochondria and metabolism in neurogenesis. Curr Opin Neurobiol 69:231–40
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124(3):319–335
Kozareva DA, Cryan JF, Nolan YM (2019) Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 18(5):e13007
van Praag H et al (2002) Functional neurogenesis in the adult hippocampus. Nature 415(6875):1030–1034
Ciric T, Cahill SP, Snyder JS (2019) Dentate gyrus neurons that are born at the peak of development, but not before or after, die in adulthood. Brain Behav 9(10):e01435
Olariu A et al (2005) A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus 15(6):750–762
Dupret D et al (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5(8):e214
Gao Q et al (2022) PINK1-mediated Drp 1(S616) phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission. Signal Transduct Target Ther 7(1):103
Funding
This work is supported by the National Natural Science Foundation of China (grant number: 82171191, 81971051 to YW, 81901100 to HH) and Jiangsu Province Special Program for Young Medical Talent (QNRC2016587 to HH).
Author information
Authors and Affiliations
Contributions
Project conception: HH, YW, CZ, HZ
Study design: HH, YW, CZ, HZ
Performance of experiments: HH, NW, JL, YQ, WW, QL, CC,
Initial data collection and analysis: HW, YL, WD, JW
Final data analysis: HW, YL, WD, JW
Writing of paper: HH, YW, CZ, HZ
Corresponding authors
Ethics declarations
Ethics Approval
The experimental protocol was approved by the Institutional Animal Care and Ethics Committee of Xuzhou Medical University and in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council.
Consent to Participate
Not applicable.
Consent for Publication
Yes.
Conflict of Interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Wang, N., Lin, JT. et al. Repeated Ketamine Anesthesia during the Neonatal Period Impairs Hippocampal Neurogenesis and Long-Term Neurocognitive Function by Inhibiting Mfn2-Mediated Mitochondrial Fusion in Neural Stem Cells. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03921-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03921-2