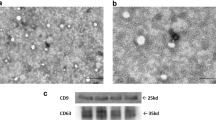
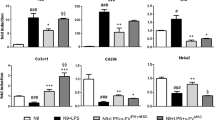
Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease, with sporadic form being the predominant type. Neuroinflammation plays a critical role in accelerating pathogenic processes in AD. Mesenchymal stem cell (MSC)–derived small extracellular vesicles (MSC-sEVs) regulate inflammatory responses and show great promise for treating AD. Induced pluripotent stem cell (iPSC)–derived MSCs are similar to MSCs and exhibit low immunogenicity and heterogeneity, making them promising cell sources for clinical applications. This study examined the anti-inflammatory effects of MSC-sEVs in a streptozotocin-induced sporadic mouse model of AD (sAD). The intracisternal administration of iPSC-MSC-sEVs alleviated NLRP3/GSDMD-mediated neuroinflammation, decreased amyloid deposition and neuronal apoptosis, and mitigated cognitive dysfunction. Furthermore, it explored the role of miR-223-3p in the iPSC-MSC-sEVs-mediated anti-inflammatory effects in vitro. miR-223-3p directly targeted NLRP3, whereas inhibiting miR-223-3p almost completely reversed the suppression of NLRP3 by MSC-sEVs, suggesting that miR-223-3p may, at least partially, account for MSC-sEVs-mediated anti-inflammation. Results obtained suggest that intracisternal administration of iPSC-MSC-sEVs can reduce cognitive impairment by inhibiting NLRP3/GSDMD neuroinflammation in a sAD mouse model. Therefore, the present study provides a proof-of-principle for applying iPSC-MSC-sEVs to target neuroinflammation in sAD.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Ferrari C, Sorbi S (2021) The complexity of Alzheimer’s disease: an evolving puzzle. Physiol Rev 101:1047–1081. https://doi.org/10.1152/physrev.00015.2020
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17:157–172. https://doi.org/10.1038/s41582-020-00435-y
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18:2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De Strooper B et al (2023) Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 145:175–195. https://doi.org/10.1007/s00401-022-02528-y
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. https://doi.org/10.1038/nature11729
Lonnemann N, Hosseini S, Marchetti C, Skouras DB, Stefanoni D, D’Alessandro A, Dinarello CA, Korte M (2020) The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 117:32145–32154. https://doi.org/10.1073/pnas.2009680117
Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA et al (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316. https://doi.org/10.1016/j.bbi.2016.12.014
Shi B, Zhao J, Xu Z, Chen C, Xu L, Xu C, Sun M, Kuang H (2022) Chiral nanoparticles force neural stem cell differentiation to alleviate Alzheimer’s disease. Adv Sci (Weinh) 9:e2202475. https://doi.org/10.1002/advs.202202475
Wang ZB, Wang ZT, Sun Y, Tan L, Yu JT (2022) The future of stem cell therapies of Alzheimer’s disease. Ageing Res Rev 80:101655. https://doi.org/10.1016/j.arr.2022.101655
Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C et al (2013) Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res Ther 4:76. https://doi.org/10.1186/scrt227
Xu F, Fei Z, Dai H, Xu J, Fan Q, Shen S, Zhang Y, Ma Q et al (2022) Mesenchymal stem cell-derived extracellular vesicles with high PD-L1 expression for autoimmune diseases treatment. Adv Mater 34:e2106265. https://doi.org/10.1002/adma.202106265
Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, Oertel FC, Scheel M et al (2020) Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 143:3574–3588. https://doi.org/10.1093/brain/awaa333
Markoutsa E, Mayilsamy K, Gulick D, Mohapatra SS, Mohapatra S (2022) Extracellular vesicles derived from inflammatory-educated stem cells reverse brain inflammation-implication of miRNAs. Mol Ther 30:816–830. https://doi.org/10.1016/j.ymthe.2021.08.008
Brennan MA, Layrolle P, Mooney DJ (2020) Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater 30. https://doi.org/10.1002/adfm.201909125
Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L et al (2020) Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl Med 9:1068–1084. https://doi.org/10.1002/sctm.19-0327
Peng H, Li Y, Ji W, Zhao R, Lu Z, Shen J, Wu Y, Wang J et al (2022) Intranasal administration of self-oriented nanocarriers based on therapeutic exosomes for synergistic treatment of Parkinson’s disease. ACS Nano 16:869–884. https://doi.org/10.1021/acsnano.1c08473
Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T, Ashery U, Popovtzer R et al (2019) Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett 19:3422–3431. https://doi.org/10.1021/acs.nanolett.8b04148
Drummond E, Wisniewski T (2017) Alzheimer’s disease: experimental models and reality. Acta Neuropathol 133:155–175. https://doi.org/10.1007/s00401-016-1662-x
Zhang L, Chen C, Mak MS, Lu J, Wu Z, Chen Q, Han Y, Li Y et al (2020) Advance of sporadic Alzheimer’s disease animal models. Med Res Rev 40:431–458. https://doi.org/10.1002/med.21624
Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 108:21–43. https://doi.org/10.1016/j.pneurobio.2013.06.004
Fan M, Liu S, Sun HM, Ma MD, Gao YJ, Qi CC, Xia QR, Ge JF (2022) Bilateral intracerebroventricular injection of streptozotocin induces AD-like behavioral impairments and neuropathological features in mice: involved with the fundamental role of neuroinflammation. Biomed Pharmacother 153:113375. https://doi.org/10.1016/j.biopha.2022.113375
Nemeth K, Bayraktar R, Ferracin M, Calin GA (2023) Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. https://doi.org/10.1038/s41576-023-00662-1
Yin Z, Herron S, Silveira S, Kleemann K, Gauthier C, Mallah D, Cheng Y, Margeta MA et al (2023) Identification of a protective microglial state mediated by miR-155 and interferon-gamma signaling in a mouse model of Alzheimer’s disease. Nat Neurosci 26:1196–1207. https://doi.org/10.1038/s41593-023-01355-y
Walgrave H, Balusu S, Snoeck S, Vanden Eynden E, Craessaerts K, Thrupp N, Wolfs L, Horre K et al (2021) Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease. Cell Stem Cell 28:1805-1821 e8. https://doi.org/10.1016/j.stem.2021.05.001
Gao WX, Sun YQ, Shi JB, Li CL, Fang SB, Wang D, Deng XQ, Wen WP et al (2017) Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther 8:16. https://doi.org/10.1186/s13287-017-0499-0
Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, Peng YQ, Xu ZB et al (2020) Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles 9:1723260. https://doi.org/10.1080/20013078.2020.1723260
Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF (2022) Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation 19:35. https://doi.org/10.1186/s12974-022-02393-2
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2012) Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 33(196):e29-40. https://doi.org/10.1016/j.neurobiolaging.2010.05.027
Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, Li X, Song N et al (2021) NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 6. https://doi.org/10.1172/jci.insight.146852
Jayaraman A, Htike TT, James R, Picon C, Reynolds R (2021) TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol Commun 9:159. https://doi.org/10.1186/s40478-021-01264-w
Gerrits E, Brouwer N, Kooistra SM, Woodbury ME, Vermeiren Y, Lambourne M, Mulder J, Kummer M et al (2021) Distinct amyloid-beta and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol 141:681–696. https://doi.org/10.1007/s00401-021-02263-w
Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y et al (2018) Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res 43:2165–2177. https://doi.org/10.1007/s11064-018-2641-5
Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, Liu JS, Dong YR et al (2019) RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing 16:10. https://doi.org/10.1186/s12979-019-0150-2
Bodart-Santos V, de Carvalho LRP, de Godoy MA, Batista AF, Saraiva LM, Lima LG, Abreu CA, De Felice FG et al (2019) Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Stem Cell Res Ther 10:332. https://doi.org/10.1186/s13287-019-1432-5
Abu Nasra NO, Elzayat EM, Dawood KM, Hagag NM, Yehya MM, Hosney M (2022) Regulatory effect of adipose-derived mesenchymal stem cells and/or acitretin on Adam10 gene in Alzheimer’s disease rat model. Curr Stem Cell Res Ther 17:370–388. https://doi.org/10.2174/1574888X17666220302154618
Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG et al (2008) Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 9:60. https://doi.org/10.1186/1471-2121-9-60
Gao WX, Sun YQ, Shi J, Li CL, Fang SB, Wang D, Deng XQ, Wen W et al (2017) Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther 8:48. https://doi.org/10.1186/s13287-017-0499-0
Siddiqui N, Ali J, Parvez S, Najmi AK, Akhtar M (2023) Neuroprotective role of DPP-4 inhibitor linagliptin against neurodegeneration, neuronal insulin resistance and neuroinflammation induced by intracerebroventricular streptozotocin in rat model of Alzheimer’s disease. Neurochem Res 48:2714–2730. https://doi.org/10.1007/s11064-023-03924-w
Liu P, Chen W, Kang Y, Wang C, Wang X, Liu W, Hayashi T, Qiu Z et al (2023) Silibinin ameliorates STING-mediated neuroinflammation via downregulation of ferroptotic damage in a sporadic Alzheimer’s disease model. Arch Biochem Biophys 744:109691. https://doi.org/10.1016/j.abb.2023.109691
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, Partoazar A (2020) Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci 255:117861. https://doi.org/10.1016/j.lfs.2020.117861
Kimiz-Gebologlu I, Oncel SS (2022) Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release 347:533–543. https://doi.org/10.1016/j.jconrel.2022.05.027
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779. https://doi.org/10.1038/mt.2011.164
Zhang L, Wu T, Shan Y, Li G, Ni X, Chen X, Hu X, Lin L et al (2021) Therapeutic reversal of Huntington’s disease by in vivo self-assembled siRNAs. Brain 144:3421–3435. https://doi.org/10.1093/brain/awab354
Wang C, Borger V, Mohamud Yusuf A, Tertel T, Stambouli O, Murke F, Freund N, Kleinschnitz C et al (2022) Postischemic neuroprotection associated with anti-inflammatory effects by mesenchymal stromal cell-derived small extracellular vesicles in aged mice. Stroke 53:e14–e18. https://doi.org/10.1161/STROKEAHA.121.035821
You HJ, Fang SB, Wu TT, Zhang H, Feng YK, Li XJ, Yang HH, Li G et al (2020) Mesenchymal stem cell-derived exosomes improve motor function and attenuate neuropathology in a mouse model of Machado-Joseph disease. Stem Cell Res Ther 11:222. https://doi.org/10.1186/s13287-020-01727-2
Choi H, Kim MY, Kim DH, Yun H, Oh BK, Kim SB, Song IH, Park HS et al (2022) Quantitative biodistribution and pharmacokinetics study of GMP-grade exosomes labeled with (89)Zr radioisotope in mice and rats. Pharmaceutics 14. https://doi.org/10.3390/pharmaceutics14061118
Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T, Schaffer DV, Goodwill PW et al (2016) Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics 6:291–301. https://doi.org/10.7150/thno.13728
Sadekar SS, Bowen M, Cai H, Jamalian S, Rafidi H, Shatz-Binder W, Lafrance-Vanasse J, Chan P et al (2022) Translational approaches for brain delivery of biologics via cerebrospinal fluid. Clin Pharmacol Ther 111:826–834. https://doi.org/10.1002/cpt.2531
Mestre H, Verma N, Greene TD, Lin LA, Ladron-de-Guevara A, Sweeney AM, Liu G, Thomas VK et al (2022) Periarteriolar spaces modulate cerebrospinal fluid transport into brain and demonstrate altered morphology in aging and Alzheimer’s disease. Nat Commun 13:3897. https://doi.org/10.1038/s41467-022-31257-9
Wu TT, Su FJ, Feng YQ, Liu B, Li MY, Liang FY, Li G, Li XJ et al (2020) Mesenchymal stem cells alleviate AQP-4-dependent glymphatic dysfunction and improve brain distribution of antisense oligonucleotides in BACHD mice. Stem Cells 38:218–230. https://doi.org/10.1002/stem.3103
Blomqvist KJ, Skogster MOB, Kurkela MJ, Rosenholm MP, Ahlstrom FHG, Airavaara MT, Backman JT, Rauhala PV et al (2022) Systemic hypertonic saline enhances glymphatic spinal cord delivery of lumbar intrathecal morphine. J Control Release 344:214–224. https://doi.org/10.1016/j.jconrel.2022.03.022
Lilius TO, Blomqvist K, Hauglund NL, Liu G, Staeger FF, Baerentzen S, Du T, Ahlstrom F et al (2019) Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release 304:29–38. https://doi.org/10.1016/j.jconrel.2019.05.005
Viola KL, Klein WL (2015) Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 129:183–206. https://doi.org/10.1007/s00401-015-1386-3
Akhtar A, Dhaliwal J, Sah SP (2021) 7,8-Dihydroxyflavone improves cognitive functions in ICV-STZ rat model of sporadic Alzheimer’s disease by reversing oxidative stress, mitochondrial dysfunction, and insulin resistance. Psychopharmacology 238:1991–2009. https://doi.org/10.1007/s00213-021-05826-7
Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B et al (2018) TREM2 is a receptor for beta-amyloid that mediates microglial function. Neuron 97:1023-1031 e7. https://doi.org/10.1016/j.neuron.2018.01.031
Xie Z, Meng J, Wu Z, Nakanishi H, Hayashi Y, Kong W, Lan F, Narengaowa et al (2022) The dual nature of microglia in Alzheimer's disease: a microglia-neuron crosstalk perspective. Neuroscientist:10738584211070273. https://doi.org/10.1177/10738584211070273
Kim DW, Tu KJ, Wei A, Lau AJ, Gonzalez-Gil A, Cao T, Braunstein K, Ling JP et al (2022) Amyloid-beta and tau pathologies act synergistically to induce novel disease stage-specific microglia subtypes. Mol Neurodegener 17:83. https://doi.org/10.1186/s13024-022-00589-x
Kazkayasi I, Telli G, Nemutlu E, Uma S (2022) Intranasal metformin treatment ameliorates cognitive functions via insulin signaling pathway in ICV-STZ-induced mice model of Alzheimer’s disease. Life Sci 299:120538. https://doi.org/10.1016/j.lfs.2022.120538
Pandey A, Shen C, Feng S, Man SM (2021) Cell biology of inflammasome activation. Trends Cell Biol 31:924–939. https://doi.org/10.1016/j.tcb.2021.06.010
Friker LL, Scheiblich H, Hochheiser IV, Brinkschulte R, Riedel D, Latz E, Geyer M, Heneka MT (2020) beta-Amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Rep 30:3743-3754 e6. https://doi.org/10.1016/j.celrep.2020.02.025
Gold M, El Khoury J (2015) beta-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin Immunopathol 37:607–611. https://doi.org/10.1007/s00281-015-0518-0
He XF, Xu JH, Li G, Li MY, Li LL, Pei Z, Zhang LY, Hu XQ (2020) NLRP3-dependent microglial training impaired the clearance of amyloid-beta and aggravated the cognitive decline in Alzheimer’s disease. Cell Death Dis 11:849. https://doi.org/10.1038/s41419-020-03072-x
Cai X, Zhang ZY, Yuan JT, Ocansey DKW, Tu Q, Zhang X, Qian H, Xu WR et al (2021) hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther 12:416. https://doi.org/10.1186/s13287-021-02492-6
Wang Y, Liu J, Wang H, Lv S, Liu Q, Li S, Yang X, Liu G (2023) Mesenchymal stem cell-derived exosomes ameliorate diabetic kidney disease through the NLRP3 signaling pathway. Stem Cells 41:368–383. https://doi.org/10.1093/stmcls/sxad010
Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C et al (2021) Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol 341:113700. https://doi.org/10.1016/j.expneurol.2021.113700
Ge X, Guo M, Hu T, Li W, Huang S, Yin Z, Li Y, Chen F et al (2020) Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther 28:503–522. https://doi.org/10.1016/j.ymthe.2019.11.017
Kong X, Gao M, Liu Y, Zhang P, Li M, Ma P, Shang P, Wang W et al (2022) GSDMD-miR-223-NLRP3 axis involved in B(a)P-induced inflammatory injury of alveolar epithelial cells. Ecotoxicol Environ Saf 232:113286. https://doi.org/10.1016/j.ecoenv.2022.113286
Zhao J, Wang H, Dong L, Sun S, Li L (2019) miRNA-20b inhibits cerebral ischemia-induced inflammation through targeting NLRP3. Int J Mol Med 43:1167–1178. https://doi.org/10.3892/ijmm.2018.4043
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367. https://doi.org/10.1126/science.aau6977
Tavasolian F, Hosseini AZ, Soudi S, Naderi M (2020) miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther 20:297–312. https://doi.org/10.2174/1566523220666200916120708
Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, Wang Y, Wang H et al (2021) Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther 12:2. https://doi.org/10.1186/s13287-020-02030-w
Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST, Wu M, Liu D et al (2021) Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 11:5248–5266. https://doi.org/10.7150/thno.54550
Xia Y, Zhou K, Sun M, Shu R, Qian J, Xie Y (2021) The miR-223-3p regulates pyroptosis through NLRP3-caspase 1-GSDMD signal axis in periodontitis. Inflammation 44:2531–2542. https://doi.org/10.1007/s10753-021-01522-y
Ren Y, Feng J, Lin Y, Reinach PS, Liu Y, Xia X, Ma X, Chen W et al (2022) MiR-223 inhibits hyperosmolarity-induced inflammation through downregulating NLRP3 activation in human corneal epithelial cells and dry eye patients. Exp Eye Res 220:109096. https://doi.org/10.1016/j.exer.2022.109096
Sun Z, Gao Z, Wu J, Zheng X, Jing S, Wang W (2022) MSC-derived extracellular vesicles activate mitophagy to alleviate renal ischemia/reperfusion injury via the miR-223-3p/NLRP3 axis. Stem Cells Int 2022:6852661. https://doi.org/10.1155/2022/6852661
Funding
This study was supported by grants from the National Key R&D Program of China (2022YFA1104900), the National Natural Science Foundation of China (No. 82271266, No. 82071255, No. 81873751, No. 82271144, No. 81970863), Guangdong Basic and Applied Basic Research Foundation (No. 2021B1515120062), the Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases (2020B1212060017), Guangdong Provincial Clinical Research Center for Neurological Diseases (2020B1111170002), Southern China International Joint Research Center for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003 and 2020A0505020004), Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, Guangzhou Clinical Research and Translational Center for Major Neurological Diseases (201604020010), Young Talent Recruitment Project of Guangdong (2019QN01Y139), The Fundamental Research Funds for the Central Universities, Sun Yat-sen University (22ykqb07), Guangdong Basic and Applied Basic Research Foundation (2021B1515120062), and Guangzhou Key R&D Program (2023B03J1233, 20220600003).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by LL, LH, SH, WC, HH, LC, FS, XL, KY, QJ, and CL. The first draft of the manuscript was written by LL, WWS, QF, and ZP. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Authors obtained ethics approval form the Sun Yat-sen University. Care of the animals was in accordance with the Guide for the Care and Use of Laboratory Animals of Sun Yat-sen University (Guangzhou, China).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, L., Huang, L., Huang, S. et al. MSC-Derived Extracellular Vesicles Alleviate NLRP3/GSDMD-Mediated Neuroinflammation in Mouse Model of Sporadic Alzheimer’s Disease. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03914-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03914-1