Abstract
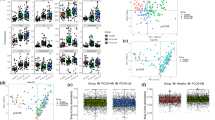
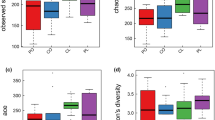
Depression is one of the complications in patients with polycystic ovary syndrome (PCOS) that leads to considerable mental health. Accumulating evidence suggests that human gut microbiomes are associated with the progression of PCOS and depression. However, whether microbiota influences depression development in PCOS patients is still uncharacterized. In this study, we employed metagenomic sequencing and transcriptome sequencing (RNA-seq) to profile the composition of the fecal microbiota and gene expression of peripheral blood mononuclear cells in depressed women with PCOS (PCOS-DP, n = 27) in comparison to mentally healthy women with PCOS (PCOS, n = 18) and compared with healthy control (HC, n = 27) and patients with major depressive disorder (MDD, n = 29). Gut microbiota assessment revealed distinct patterns in the relative abundance in the PCOS-DP compared to HC, MDD, and PCOS groups. Several gut microbes exhibited uniquely and significantly higher abundance in the PCOS-DP compared to PCOS patients, inducing EC Ruminococcus torques, Coprococcus comes, Megasphaera elsdenii, Acidaminococcus intestini, and Barnesiella viscericola. Bacteroides eggerthii was a potential gut microbial biomarker for the PCOS-DP. RNA-seq profiling identified that 35 and 37 genes were significantly elevated and downregulated in the PCOS-DP, respectively. The enhanced differential expressed genes (DEGs) in the PCOS-DP were enriched in pathways involved in signal transduction and endocrine and metabolic diseases, whereas several lipid metabolism pathways were downregulated. Intriguingly, genes correlated with the gut microbiota were found to be significantly enriched in pathways of neurodegenerative diseases and the immune system, suggesting that changes in the microbiota may have a systemic impact on the expression of neurodegenerative diseases and immune genes. Gut microbe–related DEGs of CREB3L3 and CCDC173 were possible molecular biomarkers and therapeutic targets of women with PCOS-DP. Our multi-omics data indicate shifts in the gut microbiome and host gene regulation in PCOS patients with depression, which is of possible etiological and diagnostic importance.






Similar content being viewed by others
Data Availability
Raw sequence datasets of metagenome and mRNA were deposited in the NCBI Sequence Read Archive (BioProject ID PRJNA994693).
References
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, Lizneva D, Natterson-Horowtiz B et al (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2(1):16057. https://doi.org/10.1038/nrdp.2016.57
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7(4):219–231. https://doi.org/10.1038/nrendo.2010.217
Lindholm A, Andersson L, Eliasson M, Bixo M, Sundstrom-Poromaa I (2008) Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynaecol Obstet 102(1):39–43. https://doi.org/10.1016/j.ijgo.2008.01.023
Louwers YV, Laven JS (2020) Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health 14:2633494120911038
Sadeeqa S, Mustafa T, Latif S (2018) Polycystic ovarian syndrome-related depression in adolescent girls: a review. J Pharm Bioallied Sci 10(2):55–59. https://doi.org/10.4103/JPBS.JPBS_1_18
Kolhe JV, Chhipa AS, Butani S, Chavda V, Patel SS (2022) PCOS and depression: common links and potential targets. Reprod Sci 29(11):3106–3123. https://doi.org/10.1007/s43032-021-00765-2
Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ (2019) Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol Med 49(9):1510–1520. https://doi.org/10.1017/S0033291718002076
Wang Y, Ni Z, Li K (2021) The prevalence of anxiety and depression of different severity in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol 37(12):1072–1078. https://doi.org/10.1080/09513590.2021.1942452
Sun Y, Gao S, Ye C, Zhao WJ (2023) Gut microbiota dysbiosis in polycystic ovary syndrome: mechanisms of progression and clinical applications. Front Cell Infect Microbiol 13:1142041
He F-f, Li Y-m (2020) Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res 13(1):1–13
Wu Y-X, Yang X-Y, Han B-S, Hu Y-Y, An T, Lv B-H, Lian J, Wang T-Y et al (2022) Naringenin regulates gut microbiota and SIRT1/PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Pharmacotherapy 153:113286
Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, Vivas EI et al (2017) Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Reports 18(7):1739–1750
Colldén H, Landin A, Wallenius V, Elebring E, Fändriks L, Nilsson ME, Ryberg H, Poutanen M et al (2019) The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiology-Endocrinol Metab 317(6):E1182–E1192
Wang Q, Sun Y, Zhao A, Cai X, Yu A, Xu Q, Liu W, Zhang N et al (2023) High dietary copper intake induces perturbations in the gut microbiota and affects host ovarian follicle development. Ecotoxicol Environ Saf 255:114810
Lindheim L, Bashir M, Munzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR et al (2017) Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS ONE 12(1):e0168390. https://doi.org/10.1371/journal.pone.0168390
Dong S, Jiao J, Jia S, Li G, Zhang W, Yang K, Wang Z, Liu C et al (2021) 16S rDNA full-length assembly sequencing technology analysis of intestinal microbiome in polycystic ovary syndrome. Front Cell Infect Microbiol 11:634981
Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y et al (2019) Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 25(8):1225–1233
Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, Zhong J, Lei Y et al (2013) Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics 12(1):207–214. https://doi.org/10.1074/mcp.M112.021816
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J et al (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21(6):786–796
Doll JPK, Vázquez-Castellanos JF, Schaub AC, Schweinfurth N, Kettelhack C, Schneider E, Yamanbaeva G, Mählmann L et al (2022) Fecal microbiota transplantation (FMT) as an adjunctive therapy for depression-case report. Front Psychiatry 13:815422. https://doi.org/10.3389/fpsyt.2022.815422
Green JE, Berk M, Mohebbi M, Loughman A, McGuinness AJ, Castle D, Chatterton ML, Perez J et al (2023) Feasibility, acceptability, and safety of faecal microbiota transplantation in the treatment of major depressive disorder: a pilot randomized controlled trial. Can J Psychiatry 68(5):315–326. https://doi.org/10.1177/07067437221150508
Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, Ling Y, Fu H et al (2017) Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol 8:324. https://doi.org/10.3389/fmicb.2017.00324
Jobira B, Frank DN, Pyle L, Silveira LJ, Kelsey MM, Garcia-Reyes Y, Robertson CE, Ir D et al (2020) Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota. J Clin Endocrinol Metab 105(6):e2134–e2144
Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, Lin J, Zhang Z (2019) Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol 170(1):43–52
Luan Y-y, Zhang L, Peng Y-q, Li Y-y, Liu R-x, Yin C-h (2022) Immune regulation in polycystic ovary syndrome. Clin Chim Acta 531:265–272. https://doi.org/10.1016/j.cca.2022.04.234
Bai S, Bai H, Li D, Zhong Q, Xie J, Chen JJ (2022) Gut microbiota-related inflammation factors as a potential biomarker for diagnosing major depressive disorder. Front Cell Infect Microbiol 12:831186. https://doi.org/10.3389/fcimb.2022.831186
Su NJ, Ma J, Feng DF, Zhou S, Li ZT, Zhou WP, Deng H, Liang JY et al (2018) The peripheral blood transcriptome identifies dysregulation of inflammatory response genes in polycystic ovary syndrome. Gynecol Endocrinol 34(7):584–588. https://doi.org/10.1080/09513590.2017.1418851
Du C, Chen X (2021) Transcriptome profiling of oocytes at the germinal vesicle stage from women from Mongolia with polycystic ovary syndrome. Int J Gen Med 4469–4478
Li J, Chen H, Gou M, Tian C, Wang H, Song X, Keefe DL, Bai X et al (2021) Molecular features of polycystic ovary syndrome revealed by transcriptome analysis of oocytes and cumulus cells. Front Cell Dev Biol 9:735684. https://doi.org/10.3389/fcell.2021.735684
Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, Improda FP, Verrazzo P et al (2021) Microbiome and PCOS: state-of-art and future aspects. Int J Mol Sci 22(4). https://doi.org/10.3390/ijms22042048
Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L (2016) Association between polycystic ovary syndrome and gut microbiota. PLoS ONE 11(4):e0153196
Liang Y, Ming Q, Liang J, Zhang Y, Zhang H, Shen T (2020) Gut microbiota dysbiosis in polycystic ovary syndrome: association with obesity—a preliminary report. Can J Physiol Pharmacol 98(11):803–809
Winter G, Hart RA, Charlesworth RPG, Sharpley CF (2018) Gut microbiome and depression: what we know and what we need to know. Rev Neurosci 29(6):629–643. https://doi.org/10.1515/revneuro-2017-0072
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6(4):278–296. https://doi.org/10.1111/j.2044-8260.1967.tb00530.x
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20(1):257. https://doi.org/10.1186/s13059-019-1891-0
Lu J, Breitwieser FP, Thielen P, Salzberg SL (2017) Bracken: estimating species abundance in metagenomics data. PeerJ Comput Sci 3:e104
Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, Lipson KS, Knight R et al (2018) Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods 15(11):962–968
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:1–18
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159. https://doi.org/10.1006/abio.1987.9999
Ryan FJ, Drew DP, Douglas C, Leong LE, Moldovan M, Lynn M, Fink N, Sribnaia A et al (2019) Changes in the composition of the gut microbiota and the blood transcriptome in preterm infants at less than 29 weeks gestation diagnosed with bronchopulmonary dysplasia. mSystems 4(5):e00484-00419
Goll R, Johnsen PH, Hjerde E, Diab J, Valle PC, Hilpusch F, Cavanagh JP (2020) Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes 12(1):1794263
Yap CX, Henders AK, Alvares GA, Wood DL, Krause L, Tyson GW, Restuadi R, Wallace L et al (2021) Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 184(24):5916-5931. e5917
Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T (2011) Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27(3):431–432
Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A (2007) Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril 87(6):1369–1376. https://doi.org/10.1016/j.fertnstert.2006.11.039
Kamada N, Seo S-U, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13(5):321–335
Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI (2020) Gut microbiota and immune system interactions. Microorganisms 8(10):1587
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10(11):735–742. https://doi.org/10.1038/nrmicro2876
Shoubridge AP, Choo JM, Martin AM, Keating DJ, Wong M-L, Licinio J, Rogers GB (2022) The gut microbiome and mental health: advances in research and emerging priorities. Mol Psychiatry 27(4):1908–1919. https://doi.org/10.1038/s41380-022-01479-w
Cenit MC, Sanz Y, Codoñer-Franch P (2017) Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23(30):5486
Dinan TG, Cryan JF (2017) Brain-gut-microbiota axis and mental health. Psychosom Med 79(8):920–926
Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, Chen Z-J, Du Y (2020) Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril 113(6):1286-1298.e1284. https://doi.org/10.1016/j.fertnstert.2020.01.027
Yurtdaş G, Akdevelioğlu Y (2020) A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr 39(4):371–382
Park S, Li C, Wu X, Zhang T (2023) Gut microbiota alterations and their functional differences in depression according to enterotypes in Asian individuals. Intl J Mol Sci 24(17). https://doi.org/10.3390/ijms241713329
Rong H, Xie X-h, Zhao J, Lai W-t, Wang M-b, Xu D, Liu Y-h, Guo Y-y, Xu S-x, Deng W-f, Yang Q-f, Xiao L, Zhang Y-l, He F-s, Wang S, Liu T-b (2019) Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res 113:90–99. https://doi.org/10.1016/j.jpsychires.2019.03.017
Aljumaah MR, Bhatia U, Roach J, Gunstad J, Peril MA (2022) The gut microbiome, mild cognitive impairment, and probiotics: a randomized clinical trial in middle-aged and older adults. Clin Nutr 41(11):2565–2576
Morell Miranda P, Bertolini F, Kadarmideen HN (2019) Investigation of gut microbiome association with inflammatory bowel disease and depression: a machine learning approach. F1000Research 7:702
Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD (2019) Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry 9(1):184. https://doi.org/10.1038/s41398-019-0516-4
Gałecka M, Szemraj J, Su K-P, Halaris A, Maes M, Skiba A, Gałecki P, Bliźniewska-Kowalska K (2022) Is the JAK-STAT signaling pathway involved in the pathogenesis of depression? J Clin Med 11(7). https://doi.org/10.3390/jcm11072056
Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, Trivedi MH (2017) Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: novel T cell biomarker for antidepressant medication selection. Brain Behav Immun 66:103–110. https://doi.org/10.1016/j.bbi.2017.07.005
Miller AH (2010) Depression and immunity: a role for T cells? Brain Behav Immun 24(1):1–8. https://doi.org/10.1016/j.bbi.2009.09.009
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(8):1281–1283
Parizek A, Mikesova M, Jirák R, Hill M, Koucký M, Paskova A, Velíková M, Adamcová K et al (2014) Steroid hormones in the development of postpartum depression. Physiol Res 63:S277
Pike CJ, Carroll JC, Rosario ER, Barron AM (2009) Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 30(2):239–258
Csölle C, Andó RD, Baranyi M, Haller J, Sperlágh B (2009) The role of P2X7 ATP receptors in the nervous system: potential implications in inflammatory and depression-like diseases. BMC Pharmacol 9(2):A53. https://doi.org/10.1186/1471-2210-9-S2-A53
Shen M, Song Z, Wang J-H (2019) microRNA and mRNA profiles in the amygdala are associated with stress-induced depression and resilience in juvenile mice. Psychopharmacology 236(7):2119–2142. https://doi.org/10.1007/s00213-019-05209-z
Zhang L, Li Q, Wang H, Wu Y, Ye X, Gong Z, Li Q, Xuan A (2022) Gadd45g, A novel antidepressant target, mediates metformin-induced neuronal differentiation of neural stem cells via DNA demethylation. Stem Cells 40(1):59–73. https://doi.org/10.1093/stmcls/sxab001
Pandey SS, Hendrich C, Andrade MO, Wang N (2021) Candidatus Liberibacter: from movement, host responses, to symptom development of citrus huanglongbing. Phytopathology® 112 (1):55–68. https://doi.org/10.1094/PHYTO-08-21-0354-FI
Wade WG (1996) The role of Eubacterium species in periodontal disease and other oral infections. Microb Ecol Health Dis 9(6):367–370. https://doi.org/10.3109/08910609609166480
Ghorbani M, Ferreira D, Maioli S (2023) A metagenomic study of gut viral markers in amyloid-positive Alzheimer’s disease patients. Alzheimer’s Res Ther 15(1):141. https://doi.org/10.1186/s13195-023-01285-8
Montecucco C, Rasotto MB (2015) On botulinum neurotoxin variability. mBio 6(1). https://doi.org/10.1128/mBio.02131-14
Finzi E, Wasserman E (2006) Treatment of depression with botulinum toxin A: a case series. Dermatol Surg: official publication for American Society for Dermatologic Surgery [et al] 32(5):645–649; discussion 649–650. https://doi.org/10.1111/j.1524-4725.2006.32136.x
Luvisetto S (2021) Botulinum neurotoxins in central nervous system: an overview from animal models to human therapy. Toxins 13(11).https://doi.org/10.3390/toxins13110751
Li Y, Liu T, Luo W (2021) Botulinum neurotoxin therapy for depression: therapeutic mechanisms and future perspective. Front Psychiatry 12:584416. https://doi.org/10.3389/fpsyt.2021.584416
Zhang Q, Wu W, Fan Y, Li Y, Liu J, Xu Y, Jiang C, Tang Z et al (2021) The safety and efficacy of botulinum toxin A on the treatment of depression. Brain and behavior 11(9):e2333. https://doi.org/10.1002/brb3.2333
Mayneris-Perxachs J, Castells-Nobau A, Arnoriaga-Rodríguez M, Garre-Olmo J, Puig J, Ramos R, Martínez-Hernández F, Burokas A et al (2022) Caudovirales bacteriophages are associated with improved executive function and memory in flies, mice, and humans. Cell Host Microbe 30(3):340-356.e348. https://doi.org/10.1016/j.chom.2022.01.013
Acknowledgements
We wish to thank Seqhealth Technology Co., LTD (Wuhan, China), for RNA-seq.
Funding
This work was supported by the Fujian Provincial Natural Science Foundation (grant number 2022J01279), the doctoral research project of the Second Affiliated Hospital of Fujian Medical University (grant number BS202203), and the Fujian Provincial Health Research Talents Training Project (grant number 2019–1-48).
Author information
Authors and Affiliations
Contributions
LY and JF carried out the bioinformatic analyses. XB and XC collected samples and analyzed the clinical factors. LY and MS supervised the work and wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the institutional research board. Approval was granted by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (Date 26–11-2021 /No 498).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, L., Chen, X., Bai, X. et al. Microbiota Alters and Its Correlation with Molecular Regulation Underlying Depression in PCOS Patients. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03744-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03744-7