Abstract
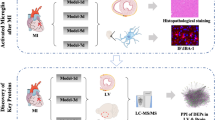
Neuroinflammation caused by microglia in the central nervous system (CNS) is observed after myocardial infarction (MI). However, the inflammatory response mechanism remains unclear. BuChang Naoxintong capsule (NXT) is a Chinese medicine for treating ischemic cardio-cerebrovascular diseases, requiring more studies to understand the pharmacodynamic mechanism. Permanent ligation of the left anterior descending coronary artery (LAD) was performed in rats. Additionally, histopathological staining in the left ventricular (LV) and immunofluorescence within the brain cortex after 1 d and 7 d of MI were performed to determine the NXT pharmacodynamic action and best administration dosage. Proteomics helped obtain the essential proteins related to neuroinflammation and MI in the heart and brain tissue after 7 d of MI. Based on TTC, HE, Masson, and immunofluorescence staining results of CD206 and IBA-1, NXT demonstrated a better pharmacodynamic action towards myocardial injury and neuroinflammation after 7 d of MI. Moreover, the human equivalent dosage of NXT (220 mg/kg) became the best administration dose. The proteome bioinformatics analysis in the LV and brain cortex was performed. Thus, the elongation of very long-chain fatty acids protein 5 (ELOVL5) and ATP-binding cassette subfamily G member 4 (ABCG4) became critical proteins related to MI and neuroinflammation. The western blotting results indicated that ABCG4 expression possessed the same trend as the proteomics results. The auto-dock results revealed that ABCG4 had a good binding ability with Ferulic acid, Paeoniflorin, and Tanshinone II A, the key ingredients of NXT. The cellular thermal shift assay results demonstrated that ABCG4 showed better thermal stability post-NXT treatment. NXT can improve myocardial injury, such as heart infarct size, pathological injury, myocardial fibrosis, and inflammatory cell infiltration. Additionally, brain neuroinflammation induced by microglia after MI affects the expression and structure of ABCG4. Thus, ABCG4 could be the key protein associated with MI and neuroinflammation.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50:e344–e418. https://doi.org/10.1161/STR.0000000000000211
van den Hurk K, Reijmer YD, van den Berg E et al (2011) Heart failure and cognitive function in the general population: the Hoorn study. Eur J Heart Fail 13:1362–1369. https://doi.org/10.1093/eurjhf/hfr138
Sampson UK, Pfeffer MA, McMurray JJV et al (2007) Predictors of stroke in high-risk patients after acute myocardial infarction: insights from the VALIANT trial. Eur Heart J 28:685–691. https://doi.org/10.1093/eurheartj/ehl197
Davidson KW (2017) Waiting for Godot: engaging in discussions about depression care in patients with acute myocardial infarction while waiting for a definitive trial that never appears. Circulation 135:1690–1692. https://doi.org/10.1161/CIRCULATIONAHA.117.027610
Bot M, Pouwer F, Zuidersma M et al (2012) Association of coexisting diabetes and depression with mortality after myocardial infarction. Diabetes Care 35:503–509. https://doi.org/10.2337/dc11-1749
Pino EC, Zuo Y, Borba CP et al (2018) Clinical depression and anxiety among ST-elevation myocardial infarction hospitalizations: results from nationwide inpatient sample 2004–2013. Psychiat Res 266:291–300. https://doi.org/10.1016/j.psychres.2018.03.025
Osteraas ND, Lee VH (2017) Neurocardiology. Handb Clin Neurol 140:49–65. https://doi.org/10.1016/B978-0-444-63600-3.00004-0
Longhurst JC (1990) Coronary arteriolar vasoconstriction in myocardial ischaemia: reflexes, sympathetic nervous system, catecholamines. Eur Heart J 11 Suppl B:43–52. https://doi.org/10.1093/eurheartj/11.suppl_b.43
Huo J-Y, Jiang W-Y, Lyu Y-T et al (2021) Renal denervation attenuates neuroinflammation in the brain by regulating gut-brain axis in rats with myocardial infarction. Front Cardiovasc Med 8:650140. https://doi.org/10.3389/fcvm.2021.650140
Gelosa P, Castiglioni L, Rzemieniec J et al (2022) Cerebral derailment after myocardial infarct: mechanisms and effects of the signaling from the ischemic heart to brain. J Mol Med (Berl) 100:23–41. https://doi.org/10.1007/s00109-021-02154-3
Roth GA, Mensah GA, Johnson CO et al (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 76:2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010
Badoer E (2010) Microglia: activation in acute and chronic inflammatory states and in response to cardiovascular dysfunction. Int J Biochem Cell Biol 42:1580–1585. https://doi.org/10.1016/j.biocel.2010.07.005
Bolte AC, Lukens JR (2021) Neuroimmune cleanup crews in brain injury. Trends Immunol 42:480–494. https://doi.org/10.1016/j.it.2021.04.003
Wu Z, Wang Z-H, Liu X et al (2020) Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog Neurobiol 185:101730. https://doi.org/10.1016/j.pneurobio.2019.101730
Smajić S, Prada-Medina CA, Landoulsi Z et al (2022) Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain 145:964–978. https://doi.org/10.1093/brain/awab446
Lu C-Y, Lu P-C, Chen P-C (2019) Utilization trends in traditional Chinese medicine for acute myocardial infarction. J Ethnopharmacol 241:112010. https://doi.org/10.1016/j.jep.2019.112010
Han J, Tan H, Duan Y et al (2019) The cardioprotective properties and the involved mechanisms of NaoXinTong capsule. Pharmacol Res 141:409–417. https://doi.org/10.1016/j.phrs.2019.01.024
Zhu M, Wei J, Li Y et al (2022) Efficacy and mechanism of Buyang Huanwu decoction in patients with ischemic heart failure: a randomized, double-blind, placebo-controlled trial combined with proteomic analysis. Front Pharmacol 13:831208. https://doi.org/10.3389/fphar.2022.831208
Songsong W, Haiyu X, Yan M et al (2015) Characterization and rapid identification of chemical constituents of NaoXinTong capsules by UHPLC-linear ion trap/Orbitrap mass spectrometry. J Pharmaceut Biomed 111:104–118. https://doi.org/10.1016/j.jpba.2015.01.020
Zhang L, Chen L, You X et al (2022) Naoxintong capsule limits myocardial infarct expansion by inhibiting platelet activation through the ERK5 pathway. Phytomedicine 98:153953. https://doi.org/10.1016/j.phymed.2022.153953
Zhao J, Ouyang Y, Wang H et al (2022) An energy metabolism study on the efficacy of Naoxintong capsules against myocardial infarction in a rat model. Oxid Med Cell Longev 2022:3712500. https://doi.org/10.1155/2022/3712500
Wang Y, Yan X, Mi S et al (2017) Naoxintong attenuates Ischaemia/reperfusion Injury through inhibiting NLRP3 inflammasome activation. J Cell Mol Med 21:4–12. https://doi.org/10.1111/jcmm.12915
Xu J, Liu X, Luo L et al (2019) A metabonomics investigation into the therapeutic effects of BuChang NaoXinTong capsules on reversing the amino acid-protein interaction network of cerebral ischemia. Oxid Med Cell Longev 2019:7258624. https://doi.org/10.1155/2019/7258624
Wang X, Yin Z, Cao P et al (2021) NaoXinTong capsule ameliorates memory deficit in APP/PS1 mice by regulating inflammatory cytokines. Biomed Pharmacother 133:110964. https://doi.org/10.1016/j.biopha.2020.110964
Zhang W-J, Su W-W, Li P-B et al (2019) Naoxintong capsule Inhibits the development of cardiovascular pathological changes in Bama Minipig through improving gut microbiota. Front Pharmacol 10:1128. https://doi.org/10.3389/fphar.2019.01128
Wang M, Wang M, Zhao J et al (2023) Dengzhan Shengmai capsule attenuates cardiac fibrosis in post-myocardial infarction rats by regulating LTBP2 and TGF-β1/Smad3 pathway. Phytomedicine 116:154849. https://doi.org/10.1016/j.phymed.2023.154849
Yang Z, Berr SS, Gilson WD et al (2004) Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast-enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation 109:1161–1167. https://doi.org/10.1161/01.CIR.0000118495.88442.32
Muhammad T, Ikram M, Ullah R et al (2019) Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 11:648. https://doi.org/10.3390/nu11030648
Evonuk KS, Prabhu SD, Young ME, DeSilva TM (2017) Myocardial ischemia/reperfusion impairs neurogenesis and hippocampal-dependent learning and memory. Brain Behav Immun 61:266–273. https://doi.org/10.1016/j.bbi.2016.09.001
Thackeray JT, Hupe HC, Wang Y et al (2018) Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 71:263–275. https://doi.org/10.1016/j.jacc.2017.11.024
Yang Q-Q, Zhou J-W (2019) Neuroinflammation in the central nervous system: symphony of glial cells. Glia 67:1017–1035. https://doi.org/10.1002/glia.23571
Borst K, Dumas AA, Prinz M (2021) Microglia: immune and non-immune functions. Immunity 54:2194–2208. https://doi.org/10.1016/j.immuni.2021.09.014
Wang M, Pan W, Xu Y et al (2022) Microglia-mediated neuroinflammation: a potential target for the treatment of cardiovascular diseases. J Inflamm Res 15:3083–3094. https://doi.org/10.2147/JIR.S350109
Fang J, Little PJ, Xu S (2018) Atheroprotective effects and molecular targets of tanshinones derived from herbal medicine danshen. Med Res Rev 38:201–228. https://doi.org/10.1002/med.21438
Cheng Y-H, Lin F-H, Wang C-Y et al (2016) Recovery of oxidative stress-induced damage in Cisd2-deficient cardiomyocytes by sustained release of ferulic acid from injectable hydrogel. Biomaterials 103:207–218. https://doi.org/10.1016/j.biomaterials.2016.06.060
Mehta PM, Przyklenk K, Kloner RA (1990) Cardioprotective effects of captopril in myocardial ischaemia, ischaemia/reperfusion and infarction. Eur Heart J 11 Suppl B:94–99. https://doi.org/10.1093/eurheartj/11.suppl_b.94
Loh E, Sutton MS, Wun CC et al (1997) Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med 336:251–257. https://doi.org/10.1056/NEJM199701233360403
Rahmati M, Keshvari M, Koyanagi A et al (2023) The effectiveness of community ageing in place, advancing better living for elders as a biobehavioural environmental approach for disability among low-income older adults: a systematic review and meta-analysis. Age Ageing 52:afad053. https://doi.org/10.1093/ageing/afad053
Rahmati M, MolanouriShamsi M, Woo W et al (2023) Effects of physical rehabilitation interventions in COVID-19 patients following discharge from hospital: a systematic review. J Integr Med 21:149–158. https://doi.org/10.1016/j.joim.2023.01.003
Hoxha E, Balbo I, Parolisi R et al (2021) Elovl5 is required for proper action potential conduction along peripheral myelinated fibers. Glia 69:2419–2428. https://doi.org/10.1002/glia.24048
Hoxha E, Gabriele RMC, Balbo I et al (2017) Motor deficits and cerebellar atrophy in Elovl5 knock out mice. Front Cell Neurosci 11:343. https://doi.org/10.3389/fncel.2017.00343
Balbo I, Montarolo F, Boda E et al (2021) Elovl5 expression in the central nervous system of the adult mouse. Front Neuroanat 15:669073. https://doi.org/10.3389/fnana.2021.669073
Tachikawa M, Watanabe M, Hori S et al (2005) Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem 95:294–304. https://doi.org/10.1111/j.1471-4159.2005.03369.x
S O, C L, J R, S L (2002) ABCG4: a novel human white family ABC-transporter expressed in the brain and eye. Biochim Biophys Acta 1591. https://doi.org/10.1016/s0167-4889(02)00269-0
Westerterp M, Bochem AE, Yvan-Charvet L et al (2014) ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res 114:157–170. https://doi.org/10.1161/CIRCRESAHA.114.300738
Graham A (2015) Mitochondrial regulation of macrophage cholesterol homeostasis. Free Radic Biol Med 89:982–992. https://doi.org/10.1016/j.freeradbiomed.2015.08.010
Vaughan AM, Oram JF (2006) ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res 47:2433–2443. https://doi.org/10.1194/jlr.M600218-JLR200
Uehara Y, Yamada T, Baba Y et al (2008) ATP-binding cassette transporter G4 is highly expressed in microglia in Alzheimer’s brain. Brain Res 1217:239–246. https://doi.org/10.1016/j.brainres.2008.04.048
He Y, Su W, Chen T et al (2019) Identification of prototype compounds and derived metabolites of naoxintong capsule in beagle dog urine and feces by UFLC-Q-TOF-MS/MS. J Pharmaceut Biomed 176:112806. https://doi.org/10.1016/j.jpba.2019.112806
Wang N-Y, Li J-N, Liu W-L et al (2021) Ferulic acid ameliorates Alzheimer’s disease-like pathology and repairs cognitive decline by preventing capillary hypofunction in APP/PS1 mice. Neurotherapeutics 18:1064–1080. https://doi.org/10.1007/s13311-021-01024-7
Fan Y-X, Qian C, Liu B et al (2018) Induction of suppressor of cytokine signaling 3 via HSF-1-HSP70-TLR4 axis attenuates neuroinflammation and ameliorates postoperative pain. Brain Behav Immun 68:111–122. https://doi.org/10.1016/j.bbi.2017.10.006
Zhang L, Wei W (2020) Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther 207:107452. https://doi.org/10.1016/j.pharmthera.2019.107452
Wen J, Xu B, Sun Y et al (2019) Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res 146:104308. https://doi.org/10.1016/j.phrs.2019.104308
de Seabra Rodrigues Dias IR, Lo HH, Zhang K et al (2021) Potential therapeutic compounds from traditional Chinese medicine targeting endoplasmic reticulum stress to alleviate rheumatoid arthritis. Pharmacol Res 170:105696. https://doi.org/10.1016/j.phrs.2021.105696
Wang Y, You K, You Y et al (2022) Paeoniflorin prevents aberrant proliferation and differentiation of intestinal stem cells by controlling C1q release from macrophages in chronic colitis. Pharmacol Res 182:106309. https://doi.org/10.1016/j.phrs.2022.106309
Acknowledgements
I would first like to thank the China Academy of Chinese Medical Sciences for carrying out the study. I would like to thank my supervisor Shihuan Tang, for his guidance through each stage of the process, and also Professor Hongjun Yang, and Hongwei Wu, for their professional advice for this paper. I would like to thank my team members, Yuxin Lei, Jing Zhang, and Jing Xu, for their wonderful collaboration and support.
Funding
This work was supported by the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences(N0.CI2021A00605), the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No.CI2021B015), National Natural Science Foundation of China (No.81973711).
Author information
Authors and Affiliations
Contributions
Mengli Chang carried out the animal experiments and wrote the paper, Mengli Chang and Jing Xu conducted the analyses, Yuxin Lei and Jing Zhang carried out the picture rendering, and Shihuan Tang and Hongjun Yang conceived the framework of the paper. In the process of revising the paper, Hongwei Wu modified the language and corrected the mistakes in the paper. All authors have made a significant contribution to the work and gave final approval for the version to be published.
Corresponding authors
Ethics declarations
Ethics Approval
The experiments were approved by the China Academy of Chinese Medical Sciences' Administrative Panel on Laboratory Animal Care and performed under the institutional guidelines and ethics of the committee as part of the China Academy of Chinese Medical Sciences (ERCCACMS21-2111–02).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, M., Lei, Y., Zhang, J. et al. Effect of Naoxintong Capsule on Microglia and Proteomics of Cortex After Myocardial Infarction in Rats. Mol Neurobiol 61, 2904–2920 (2024). https://doi.org/10.1007/s12035-023-03724-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03724-x