Abstract
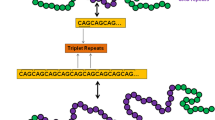
A neurodegenerative disorder (ND) refers to Huntington's disease (HD) which affects memory loss, weight loss, and movement dysfunctions such as chorea and dystonia. In the striatum and brain, HD most typically impacts medium-spiny neurons. Molecular genetics, excitotoxicity, oxidative stress (OS), mitochondrial, and metabolic dysfunction are a few of the theories advanced to explicit the pathophysiology of neuronal damage and cell death. Numerous in-depth studies of the literature have supported the therapeutic advantages of natural products in HD experimental models and other treatment approaches. This article briefly discusses the neuroprotective impacts of natural compounds against HD models. The ability of the discovered natural compounds to suppress HD was tested using either in vitro or in vivo models. Many bioactive compounds considerably lessened the memory loss and motor coordination brought on by 3-nitropropionic acid (3-NP). Reduced lipid peroxidation, increased endogenous enzymatic antioxidants, reduced acetylcholinesterase activity, and enhanced mitochondrial energy generation have profoundly decreased the biochemical change. It is significant since histology showed that therapy with particular natural compounds lessened damage to the striatum caused by 3-NP. Moreover, natural products displayed varying degrees of neuroprotection in preclinical HD studies because of their antioxidant and anti-inflammatory properties, maintenance of mitochondrial function, activation of autophagy, and inhibition of apoptosis. This study highlighted about the importance of bioactive compounds and their semi-synthetic molecules in the treatment and prevention of HD.





Similar content being viewed by others
Data Availability
No data was used for the research described in the article.
References
Krobitsch S, Kazantsev AG (2011) Huntington’s disease: from molecular basis to therapeutic advances. Int J Biochem Cell Biol 43:20–24. https://doi.org/10.1016/J.BIOCEL.2010.10.014
Kumar P, Kalonia H, Kumar A (2010) Huntington’s disease: pathogenesis to animal models. Pharmacol Rep 62:1–14. https://doi.org/10.1016/S1734-1140(10)70238-3
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR et al (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10(4):204–16. https://doi.org/10.1038/nrneurol.2014.24
Gil JM, Rego AC (2008) Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci 27:2803–2820. https://doi.org/10.1111/J.1460-9568.2008.06310.X
Bashir H (2019) Emerging therapies in Huntington’s disease. Expert Rev Neurother 19:983–995. https://doi.org/10.1080/14737175.2019.1631161
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA et al (2015) Huntington disease. Nat Rev Dis Primers 1:15005. https://doi.org/10.1038/nrdp.2015.5
Lum PT, Sekar M, Gan SH et al (2021) Protective effect of natural products against Huntington’s disease: an overview of scientific evidence and understanding Their Mechanism of Action. ACS Chem Neurosci 12:391–418. https://doi.org/10.1021/ACSCHEMNEURO.0C00824
Chiu HF, Venkatakrishnan K, Wang CK (2020) The role of nutraceuticals as a complementary therapy against various neurodegenerative diseases: a mini-review. J Tradit Complement Med 10:434–439. https://doi.org/10.1016/J.JTCME.2020.03.008
Yu M, Bega D (2019) A Review of the clinical evidence for complementary and alternative medicine in Huntington’s disease. Tremor Other Hyperkinet Mov (N Y) 9:1–9. https://doi.org/10.7916/TOHM.V0.678
Singh P, Mishra G, Molla M et al (2022) Dietary and nutraceutical-based therapeutic approaches to combat the pathogenesis of Huntington’s disease. J Funct Foods 92:105047. https://doi.org/10.1016/J.JFF.2022.105047
Evans SJW, Douglas I, Rawlins MD et al (2013) Prevalence of adult Huntington’s disease in the UK based on diagnoses recorded in general practice records. J Neurol Neurosurg Psychiatry 84:1156–1160. https://doi.org/10.1136/JNNP-2012-304636
Morrison P, Harding-Lester S, Bradley A (2011) Uptake of Huntington disease predictive testing in a complete population. Clin Genet 80:281–286. https://doi.org/10.1111/J.1399-0004.2010.01538.X
Fisher ER, Hayden MR (2014) Multisource ascertainment of Huntington disease in Canada: prevalence and population at risk. Mov Disord 29:105–114. https://doi.org/10.1002/MDS.25717
Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, Marder K, Penchaszadeh G et al (2004) Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci U S A 101:3498–3503. https://doi.org/10.1073/PNAS.0308679101
Squitieri F, Andrew SE, Goldberg YP et al (1994) DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence. Hum Mol Genet 3:2103–2114. https://doi.org/10.1093/HMG/3.12.2103
Warby SC, Montpetit A, Hayden AR et al (2009) CAG expansion in the Huntington disease gene is associated with a specific and targetable predisposing haplogroup. Am J Hum Genet 84:351–366. https://doi.org/10.1016/J.AJHG.2009.02.003
Baine FK, Kay C, Ketelaar ME et al (2013) Huntington disease in the South African population occurs on diverse and ethnically distinct genetic haplotypes. Eur J Hum Genet 21:1120–1127. https://doi.org/10.1038/EJHG.2013.2
Warby SC, Visscher H, Collins JA et al (2011) HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet 19:561–566. https://doi.org/10.1038/EJHG.2010.229
Kay C, Collins JA, Miedzybrodzka Z et al (2016) Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology 87:282–288. https://doi.org/10.1212/WNL.0000000000002858
Novak MJU, Tabrizi SJ (2010) Huntington’s disease. BMJ 340:34–40. https://doi.org/10.1136/BMJ.C3109
Roos RAC (2010) Huntington’s disease: a clinical review. Orphanet J Rare Dis 5. https://doi.org/10.1186/1750-1172-5-40
Chao TK, Hu J, Pringsheim T (2017) Risk factors for the onset and progression of Huntington disease. Neurotoxicology 61:79–99. https://doi.org/10.1016/J.NEURO.2017.01.005
Pandey M, Mohanakumar KP (2010) Usha R (2010) Mitochondrial functional alterations in relation to pathophysiology of Huntington’s disease. J Bioenerg Biomembr 423(42):217–226. https://doi.org/10.1007/S10863-010-9288-5
Scherzinger E, Sittler A, Schweiger K et al (1999) Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci U S A 96:4604–4609. https://doi.org/10.1073/PNAS.96.8.4604
Budworth H, Harris FR, Williams P, Lee DY, Holt A, Pahnke J, Szczesny B, Acevedo-Torres et al (2015) Suppression of somatic expansion delays the onset of pathophysiology in a mouse model of Huntington’s disease. PLOS Genetics 11:e1005267. https://doi.org/10.1371/journal.pgen.1005267
Kennedy L, Evans E, Chen CM et al (2003) Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum Mol Genet 12:3359–3367. https://doi.org/10.1093/HMG/DDG352
Gonitel R, Moffitt H, Sathasivam K et al (2008) DNA instability in postmitotic neurons. Proc Natl Acad Sci U S A 105:3467–3472. https://doi.org/10.1073/PNAS.0800048105
Manley K, Shirley TL, Flaherty L, Messer A (1999) Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat Genet 23:471–473. https://doi.org/10.1038/70598
Tomé S, Manley K, Simard JP et al (2013) MSH3 polymorphisms and protein levels affect CAG repeat instability in Huntington’s disease mice. PLoS Genet 9. https://doi.org/10.1371/JOURNAL.PGEN.1003280
Pinto RM, Dragileva E, Kirby A et al (2013) Mismatch repair genes Mlh1 and Mlh3 modify CAG instability in Huntington’s disease mice: genome-wide and candidate approaches. PLoS Genet 9. https://doi.org/10.1371/JOURNAL.PGEN.1003930
Wheeler VC, Lebel LA, Vrbanac V et al (2003) Mismatch repair gene Msh2 modifies the timing of early disease in Hdh(Q111) striatum. Hum Mol Genet 12:273–281. https://doi.org/10.1093/HMG/DDG056
Moffitt H, McPhail GD, Woodman B et al (2009) Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington’s disease. PLoS One 4. https://doi.org/10.1371/JOURNAL.PONE.0008025
Frost B, Diamond MI (2010) Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11:155–159. https://doi.org/10.1038/NRN2786
Pecho-Vrieseling E, Rieker C, Fuchs S et al (2014) Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci 17:1064–1072. https://doi.org/10.1038/NN.3761
Kazantsev A, Preisinger E, Dranovsky A et al (1999) Insoluble detergent-resistant aggregates form between pathological and nonpathological lengths of polyglutamine in mammalian cells. Proc Natl Acad Sci U S A 96:11404–11409. https://doi.org/10.1073/PNAS.96.20.11404
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319:916–919. https://doi.org/10.1126/SCIENCE.1141448
Labbadia J, Morimoto RI (2013) Huntington’s disease: underlying molecular mechanisms and emerging concepts. Trends Biochem Sci 38:378–385. https://doi.org/10.1016/J.TIBS.2013.05.003
Martin DDO, Ladha S, Ehrnhoefer DE, Hayden MR (2015) Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci 38:26–35. https://doi.org/10.1016/J.TINS.2014.09.003
Labbadia J, Cunliffe H, Weiss A et al (2011) Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J Clin Invest 121:3306–3319. https://doi.org/10.1172/JCI57413
Simmons DA, Rex CS, Palmer L et al (2009) Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc Natl Acad Sci U S A 106:4906–4911. https://doi.org/10.1073/PNAS.0811228106/SUPPL_FILE/0811228106SI.PDF
Burillo A, Jurcau A (2022) Molecular pathophysiological mechanisms in Huntington’s disease. Biomedicines 10:1432. https://doi.org/10.3390/BIOMEDICINES10061432
Reddy PH, Shirendeb UP (2012) Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim Biophys Acta 1822:101–110. https://doi.org/10.1016/J.BBADIS.2011.10.016
Vidal R, Caballero B, Couve A, Hetz C (2011) Converging pathways in the occurrence of endoplasmic reticulum (ER) stress in Huntington’s disease. Curr Mol Med 11:1–12. https://doi.org/10.2174/156652411794474419
Kim M, Lee HS, LaForet G et al (1999) Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci 19:964–973. https://doi.org/10.1523/JNEUROSCI.19-03-00964.1999
Johri A, Chandra A, Beal MF (2013) PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med 62:37–46. https://doi.org/10.1016/J.FREERADBIOMED.2013.04.016
Nithianantharajah J, Hannan AJ (2013) Dysregulation of synaptic proteins, dendritic spine abnormalities and pathological plasticity of synapses as experience-dependent mediators of cognitive and psychiatric symptoms in Huntington’s disease. Neuroscience 251:66–74. https://doi.org/10.1016/J.NEUROSCIENCE.2012.05.043
Ellrichmann G, Reick C, Saft C, Linker RA (2013) The role of the immune system in Huntington’s disease. Clin Dev Immunol 2013. https://doi.org/10.1155/2013/541259
Wang N, Gray M, Lu XH et al (2014) Neuronal targets for reducing mutant huntingtin expression to ameliorate disease in a mouse model of Huntington’s disease. Nat Med 20:536–541. https://doi.org/10.1038/nm.3514
Schaffar G, Breuer P, Boteva R et al (2004) Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell 15:95–105. https://doi.org/10.1016/J.MOLCEL.2004.06.029
Jana NR, Zemskov EA, Wang GH, Nukina N (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10:1049–1059. https://doi.org/10.1093/HMG/10.10.1049
Bennett EJ, Shaler TA, Woodman B et al (2007) Global changes to the ubiquitin system in Huntington’s disease. Nature 448:704–708. https://doi.org/10.1038/NATURE06022
Gunawardena S, Goldstein LSB (2005) Polyglutamine diseases and transport problems: deadly traffic jams on neuronal highways. Arch Neurol 62:46–51. https://doi.org/10.1001/ARCHNEUR.62.1.46
Liévens JC, Woodman B, Mahal A, Bates GP (2002) Abnormal phosphorylation of synapsin I predicts a neuronal transmission impairment in the R6/2 Huntington’s disease transgenic mice. Mol Cell Neurosci 20:638–648. https://doi.org/10.1006/MCNE.2002.1152
Li JY, Plomann M, Brundin P (2003) Huntington’s disease: a synaptopathy? Trends Mol Med 9:414–420. https://doi.org/10.1016/J.MOLMED.2003.08.006
Sun Y, Savanenin A, Reddy PH, Liu YF (2001) Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem 276:24713–24718. https://doi.org/10.1074/JBC.M103501200
Almeida S, Domingues A, Rodrigues L et al (2004) FK506 prevents mitochondrial-dependent apoptotic cell death induced by 3-nitropropionic acid in rat primary cortical cultures. Neurobiol Dis 17:435–444. https://doi.org/10.1016/J.NBD.2004.07.002
Choo YS, Johnson GVW, MacDonald M et al (2004) Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13:1407–1420. https://doi.org/10.1093/HMG/DDH162
Aiken CT, Steffan JS, Guerrero CM et al (2009) Phosphorylation of threonine 3: implications for Huntingtin aggregation and neurotoxicity. J Biol Chem 284:29427–29436. https://doi.org/10.1074/JBC.M109.013193
Sahoo B, Arduini I, Drombosky KW, Kodali R, Sanders LH, Greenamyre JT, Wetzel R (2016) Folding landscape of mutant huntingtin exon1: diffusible multimers, oligomers and fibrils, and no detectable monomer. PLoS One 11(6):e0155747. https://doi.org/10.1371/journal.pone.0155747
Moore BA, Barnett JE (2017) Normal function of huntingtin. Case Stud Clin Psychol Sci Bridg Gap Sci Pract: 243–273. https://doi.org/10.1093/MED/9780199929146.003.0011
Atwal RS, Xia J, Pinchev D et al (2007) Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet 16:2600–2615. https://doi.org/10.1093/HMG/DDM217
Tam S, Spiess C, Auyeung W et al (2009) The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol 16:1279–1285. https://doi.org/10.1038/NSMB.1700
Cornett J, Cao F, Wang CE et al (2005) Polyglutamine expansion of huntingtin impairs its nuclear export. Nat Genet 37:198–204. https://doi.org/10.1038/NG1503
Steffan JS, Agrawal N, Pallos J et al (2004) SUMO modification of Huntingtin and Huntington’s disease pathology. Science 304:100–104. https://doi.org/10.1126/SCIENCE.1092194
Thakur AK, Jayaraman M, Mishra R et al (2009) Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol 16:380–389. https://doi.org/10.1038/NSMB.1570
Jayaraman M, Kodali R, Sahoo B, Thakur AK, Mayasundari A, Mishra R, Peterson CB, Wetzel R et al (2012) Slow amyloid nucleation via α-helix-rich oligomeric intermediates in short polyglutamine-containing huntingtin fragments. J Mol Biol 415:881–899. https://doi.org/10.1016/J.JMB.2011.12.010
Scherzinger E, Lurz R, Turmaine M et al (1997) Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90:549–558. https://doi.org/10.1016/S0092-8674(00)80514-0
Wanker EE (2000) Protein aggregation and pathogenesis of Huntington’s disease: mechanisms and correlations. Biol Chem 381:937–942. https://doi.org/10.1515/BC.2000.114/MACHINEREADABLECITATION/RIS
Wanker EE, Ast A, Schindler F et al (2019) The pathobiology of perturbed mutant huntingtin protein–protein interactions in Huntington’s disease. J Neurochem 151:507–519. https://doi.org/10.1111/JNC.14853
Michalek M, Salnikov ES, Werten S, Bechinger B (2013) Membrane interactions of the amphipathic amino terminus of huntingtin. Biochemistry 52:847–858. https://doi.org/10.1021/BI301325Q
Wetzel R (2012) Physical chemistry of polyglutamine: intriguing tales of a monotonous sequence. J Mol Biol 421:466–490. https://doi.org/10.1016/J.JMB.2012.01.030
Faber PW, Barnes GT, Srinidhi J et al (1998) Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet 7:1463–1474. https://doi.org/10.1093/HMG/7.9.1463
Caron NS, Desmond CR, Xia J, Truant R (2013) Polyglutamine domain flexibility mediates the proximity between flanking sequences in huntingtin. Proc Natl Acad Sci U S A 110:14610–14615. https://doi.org/10.1073/PNAS.1301342110
Davies SW, Turmaine M, Cozens BA et al (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548. https://doi.org/10.1016/S0092-8674(00)80513-9
DiFiglia M, Sapp E, Chase KO et al (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993. https://doi.org/10.1126/SCIENCE.277.5334.1990
Sahl SJ, Weiss LE, Duim WC et al (2012) Cellular inclusion bodies of mutant huntingtin exon 1 obscure small fibrillar aggregate species. Sci Rep 2. https://doi.org/10.1038/SREP00895
Poirier MA, Li H, Macosko J et al (2002) Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem 277:41032–41037. https://doi.org/10.1074/JBC.M205809200
Buckley NJ, Johnson R, Zuccato C, Bithell A, Cattaneo E (2010) The role of REST in transcriptional and epigenetic dysregulation in Huntington’s disease. Neurobiol Dis 39:28–39. https://doi.org/10.1016/j.nbd.2010.02.003
Marcellin D, Abramowski D, Young D et al (2012) Fragments of HdhQ150 mutant huntingtin form a soluble oligomer pool that declines with aggregate deposition upon aging. Plos One 7. https://doi.org/10.1371/JOURNAL.PONE.0044457
Chen S, Berthelier V, Yang W, Wetzel R (2001) Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol 311:173–182. https://doi.org/10.1006/JMBI.2001.4850
Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99:10417–10422. https://doi.org/10.1073/PNAS.152161099
Ossato G, Digman MA, Aiken C et al (2010) A two-step path to inclusion formation of huntingtin peptides revealed by number and brightness analysis. Biophys J 98:3078–3085. https://doi.org/10.1016/J.BPJ.2010.02.058
Chen S, Ferrone FA, Wetzel R (2002) Huntington’s disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci U S A 99:11884–11889. https://doi.org/10.1073/PNAS.182276099
Goh AMY, Wibawa P, Loi SM et al (2018) Huntington’s disease: neuropsychiatric manifestations of Huntington’s disease. Australas Psychiatry 26:366–375. https://doi.org/10.1177/1039856218791036
Wheelock VL, Tempkin T, Marder K et al (2003) Predictors of nursing home placement in Huntington disease. Neurology 60:998–1001. https://doi.org/10.1212/01.WNL.0000052992.58107.67
Imarisio S, Carmichael J, Korolchuk V et al (2008) Huntington’s disease: from pathology and genetics to potential therapies. Biochem J 412:191–209. https://doi.org/10.1042/BJ20071619
Wang H, Chen X, Li Y et al (2010) Tetrabenazine is neuroprotective in Huntington’s disease mice. Mol Neurodegener 5. https://doi.org/10.1186/1750-1326-5-18
Kumar A, Kumar V, Singh K et al (2020) Therapeutic advances for Huntington’s disease. Brain Sci 10. https://doi.org/10.3390/BRAINSCI10010043
de Tommaso M, Serpino C, Sciruicchio V (2011) Management of Huntington’s disease: role of tetrabenazine. Ther Clin Risk Manag 7:123. https://doi.org/10.2147/TCRM.S17152
Coppen EM, Roos RAC (2017) Current Pharmacological approaches to reduce chorea in Huntington’s disease. Drugs 77:29–46. https://doi.org/10.1007/S40265-016-0670-4
Rinaldi C, Wood MJA (2018) Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14:9–22. https://doi.org/10.1038/NRNEUROL.2017.148
Crotti A, Glass CK (2015) The choreography of neuroinflammation in Huntington’s disease. Trends Immunol 36:364–373. https://doi.org/10.1016/J.IT.2015.04.007
Wang YL, Liu W, Wada E et al (2005) Clinico-pathological rescue of a model mouse of Huntington’s disease by siRNA. Neurosci Res 53:241–249. https://doi.org/10.1016/J.NEURES.2005.06.021
Xia H, Mao Q, Eliason SL et al (2004) (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 108(10):816–820. https://doi.org/10.1038/nm1076
Miller VM, Xia H, Marrs GL et al (2003) Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci U S A 100:7195–7200. https://doi.org/10.1073/PNAS.1231012100/SUPPL_FILE/1012FIG5.JPG
Ravikumar B, Vacher C, Berger Z et al (2004) (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 366(36):585–595. https://doi.org/10.1038/ng1362
Berger Z, Ravikumar B, Menzies FM et al (2006) Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 15:433–442. https://doi.org/10.1093/HMG/DDI458
Sánchez I, Chi-Jie X, Peter J et al (1999) Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22:623–633. https://doi.org/10.1016/S0896-6273(00)80716-3
Wu PF, Zhang Z, Wang F, Chen JG (2010) Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin 31:1523–1531. https://doi.org/10.1038/APS.2010.186
Adams M, Gmünder F, Hamburger M (2007) Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J Ethnopharmacol 113:363–381. https://doi.org/10.1016/j.jep.2007.07.016
Isah T (2015) Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharmacogn Rev 9:140. https://doi.org/10.4103/0973-7847.162137
Nakanishi K (2005) Terpene trilactones from Gingko biloba: from ancient times to the 21st century. Bioorg Med Chem 13:4987–5000. https://doi.org/10.1016/J.BMC.2005.06.014
Mahadevan S, Park Y (2007) Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci 73:R14–R19. https://doi.org/10.1111/j.1750-3841.2007.00597.x
DeFeudis F, Drieu K (2000) Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets 1:25–58. https://doi.org/10.2174/1389450003349380
Smith JV, Luo Y (2004) Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol 64:465–472. https://doi.org/10.1007/S00253-003-1527-9
Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, Perry G (2019) Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 16(3):666-674. https://doi.org/10.1007/s13311-019-00767-8
Pietri S, Maurelli E, Drieu K, Culcasi M (1997) Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761). J Mol Cell Cardiol 29:733–742. https://doi.org/10.1006/JMCC.1996.0316
Bai J, Zhang C (2023) Metabolic interaction between biflavonoids in Ginkgo biloba leaves and tacrolimus. Biopharm Drug Dispos 44(2):157–164. https://doi.org/10.1002/bdd.2350
Mahdy HM, Tadros MG, Mohamed MR et al (2011) The effect of Ginkgo biloba extract on 3-nitropropionic acid-induced neurotoxicity in rats. 59:770–778. https://doi.org/10.1016/J.NEUINT.2011.07.012
Manisha T, Sisir Kumar M, Reetu S (2015) Rasayana as a public health tool in communicable diseases: a review. Int J Ayurvedic Med 6:1–7
Soumyanath A, Zhong Y-P, Yu X et al (2005) Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol 57:1221–1229. https://doi.org/10.1211/JPP.57.9.0018
Zainol MK, Abd-Hamid A, Yusof S, Muse R (2003) Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem 81:575–581. https://doi.org/10.1016/S0308-8146(02)00498-3
Hussin M, Abdul-Hamid A, Mohamad S et al (2007) Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem 100:535–541. https://doi.org/10.1016/J.FOODCHEM.2005.10.022
Singh B, Rastogi RP (1969) A reinvestigation of the triterpenes of Centella asiatica. Phytochemistry 8:917–921. https://doi.org/10.1016/S0031-9422(00)85884-7
Randriamampionona D, Diallo B, Rakotoniriana F et al (2007) Comparative analysis of active constituents in Centella asiatica samples from Madagascar: application for ex situ conservation and clonal propagation. Fitoterapia 78:482–489. https://doi.org/10.1016/J.FITOTE.2007.03.016
Singh B, Rastogi RP (1968) Chemical examination of Centella asiatica linn—III : Constitution of brahmic acid. Phytochemistry 7:1385–1393. https://doi.org/10.1016/S0031-9422(00)85642-3
Shinomol GK, Muralidhara (2008) Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicol 29:948–957. https://doi.org/10.1016/J.NEURO.2008.09.009
Kim YJ, Zhang D, Yang DC (2015) Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv 33:717–735. https://doi.org/10.1016/J.BIOTECHADV.2015.03.001
Sagredo O, Ruth Pazos M, Valdeolivas S, Fernández-Ruiz J (2012) Cannabinoids: novel medicines for the treatment of Huntington’s disease. Recent Pat CNS Drug Discov 7:41–48. https://doi.org/10.2174/157488912798842278
Valdeolivas S, Satta V, Pertwee RG et al (2012) Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington’s disease: role of CB1 and CB2 receptors. ACS Chem Neurosci 3:400–406. https://doi.org/10.1021/CN200114W
Sagredo O, González S, Aroyo I et al (2009) Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington’s disease. Glia 57:1154–1167. https://doi.org/10.1002/GLIA.20838
Lastres-Becker I, Bizat N, Boyer F et al (2003) Effects of cannabinoids in the rat model of Huntington’s disease generated by an intrastriatal injection of malonate. NeuroReport 14:813–816. https://doi.org/10.1097/00001756-200305060-00007
Jang JH, Son Y, Kang SS et al (2015) Neuropharmacological potential of gastrodia elata blume and its components. Evid Based Complement Alternat Med 2015. https://doi.org/10.1155/2015/309261
Tsai CF, Huang CL, Lin YL et al (2011) The neuroprotective effects of an extract of Gastrodia elata. J Ethnopharmacol 138:119–125. https://doi.org/10.1016/J.JEP.2011.08.064
Hu Y, Li C, Shen W (2014) Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 34:370–377. https://doi.org/10.1111/NEUP.12115
Doo AR, Kim SN, Hahm DH et al (2014) Gastrodia elata Blume alleviates L-DOPA-induced dyskinesia by normalizing FosB and ERK activation in a 6-OHDA-lesioned Parkinson’s disease mouse model. BMC Complement Altern Med 14. https://doi.org/10.1186/1472-6882-14-107
Wang Q, Shen L, Ma S, Chen M, Lin X, Hong Y, Liang S, Feng Y (2015) Effects of Ligusticum chuanxiong and Gastrodia elata on blood-brain barrier permeability in migraine rats. Int J Pharm Sci Res 70:421–6. https://doi.org/10.1691/ph.2015.4852
Yu SJ, Kim JR, Lee CK et al (2005) Gastrodia elata blume and an active component, p-hydroxybenzyl alcohol reduce focal ischemic brain injury through antioxidant related gene expressions. Biol Pharm Bull 28:1016–1020. https://doi.org/10.1248/BPB.28.1016
Wu LY, Chen WC, Tsai FS et al (2015) p-Hydroxybenzyl alcohol, an active phenolic ingredient of gastrodia elata, reverses the cycloheximide-induced memory deficit by activating the adrenal gland in rats. Am J Chin Med 43:1593–1604. https://doi.org/10.1142/S0192415X15500901
Manavalan A, Feng L, Sze SK et al (2012) New insights into the brain protein metabolism of Gastrodia elata-treated rats by quantitative proteomics. J Proteomics 75:2468–2479. https://doi.org/10.1016/J.JPROT.2012.02.029
Huang CL, Yang JM, Wang KC et al (2011) Gastrodia elata prevents huntingtin aggregations through activation of the adenosine A2A receptor and ubiquitin proteasome system. J Ethnopharmacol 138:162–168. https://doi.org/10.1016/J.JEP.2011.08.075
Jiang W, Wei W, Gaertig MA et al (2015) Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS One 10. https://doi.org/10.1371/JOURNAL.PONE.0134142
Chen CG, Hung TH, Lee CY et al (2014) Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS One 9:e115694. https://doi.org/10.1371/JOURNAL.PONE.0115694
Simões Pires EN, Frozza RL, Hoppe JB et al (2014) Berberine was neuroprotective against an in vitro model of brain ischemia: survival and apoptosis pathways involved. Brain Res 1557:26–33. https://doi.org/10.1016/J.BRAINRES.2014.02.021
Hua KF, Chao AC, Lin TY et al (2022) Ginsenoside compound K reduces the progression of Huntington’s disease via the inhibition of oxidative stress and overactivation of the ATM/AMPK pathway. J Ginseng Res 46:572–584. https://doi.org/10.1016/J.JGR.2021.11.003
Solanki I, Parihar P, Mansuri ML, Parihar MS (2015) Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr 6:64–72. https://doi.org/10.3945/an.114.007500
Tasset I, Agüera E, Olmo-Camacho R et al (2011) Melatonin improves 3-nitropropionic acid induced behavioral alterations and neurotrophic factors levels. Prog Neuropsychopharmacol Biol Psychiatry 35:1944–1949. https://doi.org/10.1016/J.PNPBP.2011.09.005
Nam E, Seung ML, Seong EK et al (2005) Melatonin protects against neuronal damage induced by 3-nitropropionic acid in rat striatum. Brain Res 1046:90–96. https://doi.org/10.1016/J.BRAINRES.2005.03.053
Kumar P, Kumar A (2009) Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol 47:2522–2530. https://doi.org/10.1016/J.FCT.2009.07.011
Mu S, Ouyang L, Liu B et al (2011) Protective effect of melatonin on 3-NP induced striatal interneuron injury in rats. Neurochem Int 59:224–234. https://doi.org/10.1016/J.NEUINT.2011.05.009
Tasset I, Espínola C, Medina FJ et al (2009) Neuroprotective effect of carvedilol and melatonin on 3-nitropropionic acid-induced neurotoxicity in neuroblastoma. J Physiol Biochem 65:291–296. https://doi.org/10.1007/BF03180581
Chakraborty J, Nthenge-Ngumbau DN, Rajamma U, Mohanakumar KP (2014) Melatonin protects against behavioural dysfunctions and dendritic spine damage in 3-nitropropionic acid-induced rat model of Huntington’s disease. Behav Brain Res 264:91–104. https://doi.org/10.1016/J.BBR.2014.01.048
Gopinath K, Sudhandiran G (2012) Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 227:134–143. https://doi.org/10.1016/J.NEUROSCIENCE.2012.07.060
Gopinath K, Prakash D, Sudhandiran G (2011) Neuroprotective effect of naringin, a dietary flavonoid against 3-nitropropionic acid-induced neuronal apoptosis. Neurochem Int 59:1066–1073. https://doi.org/10.1016/J.NEUINT.2011.08.022
Rong W, Wang J, Liu X et al (2012) Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem Res 37:1615–1623. https://doi.org/10.1007/S11064-012-0756-7
Gopinath K, Sudhandiran G (2016) Protective effect of naringin on 3-nitropropionic acid-induced neurodegeneration through the modulation of matrix metalloproteinases and glial fibrillary acidic protein. Can J Physiol Pharmacol 94:65–71. https://doi.org/10.1139/CJPP-2015-0035
Kulasekaran G, Ganapasam S (2015) Neuroprotective efficacy of naringin on 3-nitropropionic acid-induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol Cell Biochem 409:199–211. https://doi.org/10.1007/S11010-015-2525-9
Denny Joseph KM, Muralidhara (2013) Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 40:83–92. https://doi.org/10.1016/J.PNPBP.2012.08.018
Ehrnhoefer DE, Duennwald ML, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Lawrence Marsh J et al (2006) Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet 15:2743–2751. https://doi.org/10.1093/hmg/ddl210
Chang-Xiao L, Pei-Gen X (1992) Recent advances on ginseng research in China. J Ethnopharmacol 36:27–38. https://doi.org/10.1016/0378-8741(92)90057-X
Lu J-M, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7:293–302. https://doi.org/10.2174/157016109788340767
Radad K, Gille G, Liu L, Rausch WD (2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100:175–186. https://doi.org/10.1254/JPHS.CRJ05010X
Li M, Qian M, Jiang Q, Tan B, Yin Y, Han X (2023) Evidence of flavonoids on disease prevention. Antioxidants (Basel) 12(2):527. https://doi.org/10.3390/antiox12020527
Sadeghi N, Oveisi MR, Hajimahmoodi M et al (2010) The contents of sesamol in iranian sesame seeds. Iran J Pharm Res 0:101–105. https://doi.org/10.22037/IJPR.2010.796
Keum YS, Park KK, Lee JM et al (2000) Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett 150:41–48. https://doi.org/10.1016/S0304-3835(99)00369-9
Shimizu S, Fujii G, Takahashi M, Nakanishi R, Komiya M, Shimura M, Noma N, Onuma W et al (2014) Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in ApcMin/+ mice. J Clin Biochem Nutr 54:95–101. https://doi.org/10.3164/jcbn.13-91
Kim Y, Kim S, Markelonis G, Oh T (1998) Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J Neurosci Res 53:426–432. https://doi.org/10.1002/(sici)1097-4547(19981001)54:1%3C123::aid-jnr13%3E3.0.co;2-8
Kim JH, Kim S, Yoon IS et al (2005) Protective effects of ginseng saponins on 3-nitropropionic acid-induced striatal degeneration in rats. Neuropharmacology 48:743–756. https://doi.org/10.1016/J.NEUROPHARM.2004.12.013
Shivasharan BD, Nagakannan P, Thippeswamy BS et al (2013) Protective effect of Calendula officinalis Linn. flowers against 3-nitropropionic acid induced experimental Huntington’s disease in rats. Drug Chem Toxicol 36:466–473. https://doi.org/10.3109/01480545.2013.776583
Kaur M, Prakash A, Kalia AN (2016) Neuroprotective potential of antioxidant potent fractions from Convolvulus pluricaulis Chois. in 3-nitropropionic acid challenged rats. 19:70–78. https://doi.org/10.1179/1476830515Y.0000000022
Piwowar A, Rembiałkowska N, Rorbach-Dolata A et al (2020) Anemarrhenae asphodeloides rhizoma extract enriched in mangiferin protects PC12 cells against a neurotoxic agent-3-nitropropionic acid. 21:2510. https://doi.org/10.3390/IJMS21072510
Al-Sabahi BN, Fatope MO, Essa MM et al. Pomegranate seed oil: Effect on 3-nitropropionic acid-induced neurotoxicity in PC12 cells and elucidation of unsaturated fatty acids composition. 20:40–48. https://doi.org/10.1179/1476830514Y.0000000155
Malik J, Karan M, Dogra R (2017) Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: possible antioxidant mechanism. 55:980–990. https://doi.org/10.1080/13880209.2017.1285945
Kumar P, Kumar A (2009) Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J Med Food 12:591–600. https://doi.org/10.1089/JMF.2008.0028
Essa M, Singh V, Guizani N et al (2019) Phoenix dactylifera L. Fruits date fruit ameliorate oxidative stress in 3-NP intoxicated PC12 cells. Int J Nutr Pharmacol Neurol Dis 9:41. https://doi.org/10.4103/IJNPND.IJNPND_51_18
Shinomol GK, Ravikumar H, Muralidhara (2010) Prophylaxis with Centella asiatica confers protection to prepubertal mice against 3-nitropropionic-acid-induced oxidative stress in brain. Phyther Res 24:885–892. https://doi.org/10.1002/PTR.3042
Jang M, Lee MJ, Kim CS, Cho IH (2013) Korean red ginseng extract attenuates 3-nitropropionic acid-induced Huntington’s-like symptoms. Evid Based Complement Alternat Med 2013. https://doi.org/10.1155/2013/237207
Courtes AA, Arantes LP, Barcelos RP et al (2015) Protective effects of aqueous extract of luehea divaricata against behavioral and oxidative changes induced by 3-nitropropionic acid in rats. Evid Based Complement Alternat Med 2015. https://doi.org/10.1155/2015/723431
Bhangale JO, Acharya NS, Acharya SR (2016) Protective effect of Ficus religiosa (L.) against 3-nitropropionic acid induced Huntington disease. Orient Pharm Exp Med 16(3):165–174. https://doi.org/10.1007/S13596-016-0237-7
Lin Y, Zhao WR, Shi WT et al (2020) Pharmacological activity, pharmacokinetics, and toxicity of timosaponin AIII, a natural product isolated from Anemarrhena asphodeloides Bunge: a review. Front Pharmacol 11:764. https://doi.org/10.3389/FPHAR.2020.00764/XML/NLM
Wu J, Jeong HK, Bulin SE et al (2009) Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J Neurosci Res 87:1904–1912. https://doi.org/10.1002/JNR.22017
Bors W, Heller W, Michel C, Saran M (1990) Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 186:343–355. https://doi.org/10.1016/0076-6879(90)86128-I
Narayana KRAJ, Reddy MS, Chaluvadi MR, Krishna DR (2001) Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol 33:2–16
Prior RL, Cao G (2000) Analysis of botanicals and dietary supplements for antioxidant capacity: A review. J AOAC Int 83:950–956. https://doi.org/10.1093/jaoac/83.4.950
Smith PDC, Thomas P, Scurr JH, Dormandy JA (1988) Causes of venous ulceration: a new hypothesis. Br Med J (Clin Res Ed) 296:1726–1727. https://doi.org/10.1136/BMJ.296.6638.1726
Schroeter H, Boyd C, Spencer JPE et al (2002) MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging 23:861–880. https://doi.org/10.1016/S0197-4580(02)00075-1
López-López G, Moreno L, Cogolludo A et al (2004) Nitric oxide (NO) scavenging and NO protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol Pharmacol 65:851–859. https://doi.org/10.1124/MOL.65.4.851
Nasiri M, Gheibi Z, Miri A et al (2019) Effects of consuming date fruits (Phoenix dactylifera Linn) on gestation, labor, and delivery: an updated systematic review and meta-analysis of clinical trials. Complement Ther Med 45:71–84. https://doi.org/10.1016/J.CTIM.2019.05.017
Raso GM, Meli R, Di Carlo G et al (2001) Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci 68:921–931. https://doi.org/10.1016/S0024-3205(00)00999-1
Kumar P, Padi SSV, Naidu PS, Kumar A (2007) Cyclooxygenase inhibition attenuates 3-nitropropionic acid-induced neurotoxicity in rats: possible antioxidant mechanisms. Fundam Clin Pharmacol 21:297–306. https://doi.org/10.1111/J.1472-8206.2007.00485.X
Ishige K, Schubert D, Sagara Y (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30:433–446. https://doi.org/10.1016/S0891-5849(00)00498-6
Jayaprakash A, Sangeetha R (2015) Phytochemical screening of Punica granatum Linn. peel extracts. J Acad Ind Res 4:160
Kumar P, Kumar A (2010) Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: possible role of nitric oxide. Behav Brain Res 206:38–46. https://doi.org/10.1016/J.BBR.2009.08.028
Kumar P, Kalonia H, Kumar A (2010) Protective effect of sesamol against 3-nitropropionic acid-induced cognitive dysfunction and altered glutathione redox balance in rats. Basic Clin Pharmacol Toxicol 107:577–582. https://doi.org/10.1111/J.1742-7843.2010.00537.X
Verma PK, Raina R, Agarwal S, Kaur H (2018) Phytochemical ingredients and Pharmacological potential of Calendula officinalis Linn. Pharm Biomed Res 4:1–17. https://doi.org/10.18502/PBR.V4I2.214
Lagoa R, Lopez-Sanchez C, Samhan-Arias AK et al (2009) Kaempferol protects against rat striatal degeneration induced by 3-nitropropionic acid. J Neurochem 111:473–487. https://doi.org/10.1111/J.1471-4159.2009.06331.X
Nagpal K, Garg M, Arora D et al (2022) An extensive review on phytochemistry and pharmacological activities of Indian medicinal plant Celastrus paniculatus Willd. Phyther Res 36:1930–1951. https://doi.org/10.1002/PTR.7424
Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV (2005) Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta 355:97–104. https://doi.org/10.1016/J.CCCN.2004.12.009
Agarwal P, Sharma B, Fatima A, Jain SK (2014) An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac J Trop Biomed 4:245–252. https://doi.org/10.1016/S2221-1691(14)60240-9
Baba NH, Antoniades K, Habbal Z (1999) Effects of dietary canola, olive, and linolenic acid enriched olive oils on plasma lipids, lipid peroxidation and lipoprotein lipase activity in rats. Nutr Res 19:601–612. https://doi.org/10.1016/S0271-5317(99)00025-1
Kapadia GJ, Azuine MA, Tokuda H et al (2002) Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol Res 45:499–505. https://doi.org/10.1006/PHRS.2002.0992
Kumar P, Kalonia H, Kumar A (2009) Sesamol attenuate 3-nitropropionic acid-induced Huntington-like behavioral, biochemical, and cellular alterations in rats. J Asian Nat Prod Res 11:439–450. https://doi.org/10.1080/10286020902862194
Saha PS, Sarkar S, Jeyasri R et al (2020) In vitro propagation, phytochemical and neuropharmacological profiles of Bacopa monnieri (L.) Wettst.: a review. Plants 9:411. https://doi.org/10.3390/PLANTS9040411
Wang Y, Dan Y, Yang D et al (2014) The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol 153:42–60. https://doi.org/10.1016/J.JEP.2014.02.013
Renugadevi J, Prabu SM (2009) Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 256:128–134. https://doi.org/10.1016/J.TOX.2008.11.012
Hsu D-Z, Chien S-P, Chen K-T, Liu M-Y (2007) The effect of sesamol on systemic oxidative stress and hepatic dysfunction in acutely iron-intoxicated mice. Shock 28:596–601. https://doi.org/10.1097/shk.0b013e31804d4474
Jadiya P, Khan A, Sammi SR, Kaur S, Mir SS, Nazir A (2011) Anti-Parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem Biophys Res Commun 413:605–610. https://doi.org/10.1016/j.bbrc.2011.09.010
Ji D, Huang Zy, Fei Ch et al (2017) Comprehensive profiling and characterization of chemical constituents of rhizome of Anemarrhena asphodeloides Bge. J Chromatogr B Analyt Technol Biomed Life Sci 1060:355–366. https://doi.org/10.1016/J.JCHROMB.2017.06.032
Parvin S, Easmin D, Sheikh A et al (2015) Nutritional analysis of date fruits (Phoenix dactylifera L.) in perspective of Bangladesh. article. ajlifesci.org 3:274–278. https://doi.org/10.11648/j.ajls.20150304.14
Al Alawi R, Alhamdani MSS, Hoheisel JD, Baqi Y (2020) Antifibrotic and tumor microenvironment modulating effect of date palm fruit (Phoenix dactylifera L.) extracts in pancreatic cancer. Biomed Pharmacother 121. https://doi.org/10.1016/J.BIOPHA.2019.109522
Aliferis KA, Bernard-Perron D (2020) Cannabinomics: application of metabolomics in Cannabis (Cannabis sativa L.) research and development. Front Plant Sci 11:554. https://doi.org/10.3389/FPLS.2020.00554/XML/NLM
Ayaz Ali B SY, Sameer SD, Patel A, et al Pharmacokinetic drug food interaction study of nateglinide and pomegranate fruit juice. Iran J Diabetes Obes 12. https://doi.org/10.18502/ijdo.v12i4.5181
BenSaad LA, Kim KH, Quah CC et al (2017) Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement Alternat Med 17. https://doi.org/10.1186/S12906-017-1555-0
Muley BP, Khadabadi SS, Banarase NB (2009) Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review. Trop J Pharm Res 8:455–465. https://doi.org/10.4314/tjpr.v8i5.48090
Lim XY, Tan TYC, Rosli SHM et al (2021) Cannabis sativa subsp. sativa’s pharmacological properties and health effects: a scoping review of current evidence. PLoS One 16. https://doi.org/10.1371/JOURNAL.PONE.0245471
Zhan HD, Zhou HY, Sui YP et al (2016) The rhizome of Gastrodia elata Blume – an ethnopharmacological review. J Ethnopharmacol 189:361–385. https://doi.org/10.1016/J.JEP.2016.06.057
Malik J, Choudhary S, Kumar P (2015) Protective effect of Convolvulus pluricaulis standardized extract and its fractions against 3-nitropropionic acid-induced neurotoxicity in rats. Pharm Biol 53:1448–1457. https://doi.org/10.3109/13880209.2014.984856
Godkar PB, Gordon RK, Ravindran A, Doctor BP (2004) Celastrus paniculatus seed water soluble extracts protect against glutamate toxicity in neuronal cultures from rat forebrain. J Ethnopharmacol 93:213–219. https://doi.org/10.1016/J.JEP.2004.03.051
Wu LY, Chen WC, Tsai FS, Tsai CC, Wu CR, Lin LW (2015) p-Hydroxybenzyl alcohol, an active phenolic Ingredient of Gastrodia elata, reverses the cycloheximide-induced memory deficit by activating the adrenal gland in rats. Am J Chin Med 43(8):1593–604. https://doi.org/10.1142/S0192415X15500901
Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI (2012) Berberine: new perspectives for old remedies. Biochem Pharmacol 84:1260–1267. https://doi.org/10.1016/J.BCP.2012.07.018
Chen C-C, Hung T-H, Lee CY, Wang L-F, Wu C-H, Ke C-H, Chen S-F et al (2014) Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS ONE 9:e115694. https://doi.org/10.1371/journal.pone.0115694
Phulara SC, Shukla V, Tiwari S, Pandey R (2015) Bacopa monnieri promotes longevity in Caenorhabditis elegans under stress conditions. Pharmacogn Mag 11:410–416. https://doi.org/10.4103/0973-1296.153097
Chowdhury A, Sarkar J, Chakraborti T et al (2016) Protective role of epigallocatechin-3-gallate in health and disease: a perspective. Biomed Pharmacother 78:50–59. https://doi.org/10.1016/J.BIOPHA.2015.12.013
Kean JD, Kaufman J, Lomas J et al (2015) A randomized controlled trial investigating the effects of a special extract of Bacopa monnieri (CDRI 08) on hyperactivity and inattention in male children and adolescents: BACHI study protocol (ANZCTRN12612000827831). Nutrients 7:9931–9945. https://doi.org/10.3390/NU7125507
Benson S, Downey LA, Stough C et al (2014) An acute, double-blind, placebo-controlled cross-over study of 320 mg and 640 mg doses of Bacopa monnieri (CDRI 08) on multitasking stress reactivity and mood. Phytother Res 28:551–559. https://doi.org/10.1002/PTR.5029
Downey LA, Kean J, Nemeh F et al (2013) An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother Res 27:1407–1413. https://doi.org/10.1002/PTR.4864
Hardeland R, Pandi-Perumal SR, Cardinali DP (2006) Melatonin. Int J Biochem Cell Biol 38:313–316. https://doi.org/10.1016/J.BIOCEL.2005.08.020
Arendt J, Skene DJ (2005) Melatonin as a chronobiotic. Sleep Med Rev 9:25–39. https://doi.org/10.1016/J.SMRV.2004.05.002
Uabundit N, Wattanathorn J, Mucimapura S, Ingkaninan K (2010) Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J Ethnopharmacol 127:26–31. https://doi.org/10.1016/J.JEP.2009.09.056
Shinomol GK, Muralidhara (2011) Bacopa monnieri modulates endogenous cytoplasmic and mitochondrial oxidative markers in prepubertal mice brain. Phytomedicine 18:317–326. https://doi.org/10.1016/J.PHYMED.2010.08.005
Alam MA, Kauter K, Brown L (2013) Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 5:637–650. https://doi.org/10.3390/NU5030637
Shinomol GK, Bharath MMB, Muralidhara (2012) Pretreatment with Bacopa monnieri extract offsets 3-nitropropionic acid induced mitochondrial oxidative stress and dysfunctions in the striatum of prepubertal mouse brain. Can J Physiol Pharmacol 90:595–606. https://doi.org/10.1139/Y2012-030
Shinomol GK, Srinivas Bharath MM, Muralidhara (2012) Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: in vitro and in vivo evidences. Neurotox Res 22:102–114. https://doi.org/10.1007/S12640-011-9303-6
Turner CE, Elsohly MA, Boeren EG (1980) Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod 43:169–234. https://doi.org/10.1021/NP50008A001
Sharma P, Kumar V, Guleria P (2019) Naringin: biosynthesis and pharmaceutical applications. Indian J Pharm Sci 81:988–999. https://doi.org/10.36468/PHARMACEUTICAL-SCIENCES.596
Chagas MHN, Zuardi AW, Tumas V et al (2014) Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28:1088–1092. https://doi.org/10.1177/0269881114550355
Chagas MHN, Eckeli AL, Zuardi AW et al (2014) Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther 39:564–566. https://doi.org/10.1111/JCPT.12179
Ahmed AIA, Van Der Marck MA, Van Den Elsen GAH, Olde Rikkert MGM (2015) Cannabinoids in late-onset Alzheimer’s disease. Clin Pharmacol Ther 97:597–606. https://doi.org/10.1002/CPT.117
Sagredo O, Pazos MR, Satta V et al (2011) Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J Neurosci Res 89:1509–1518. https://doi.org/10.1002/JNR.22682
Stachowiak B, Szulc P (2021) Astaxanthin for the food industry. Molecules 26:2666. https://doi.org/10.3390/MOLECULES26092666
Ambati RR, Moi PS, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs 12:128–152. https://doi.org/10.3390/MD12010128
Galasso C, Orefice I, Pellone P et al (2018) On the neuroprotective role of astaxanthin: new perspectives? Mar Drugs 16. https://doi.org/10.3390/MD16080247
Debnath K, Pradhan N, Singh BK et al (2017) Poly(trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Appl Mater Interfaces 9:24126–24139. https://doi.org/10.1021/ACSAMI.7B06510/SUPPL_FILE/AM7B06510_LIVESLIDES.MP4
Sztretye M, Dienes B, Gönczi M et al (2019) Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longev 2019. https://doi.org/10.1155/2019/3849692
Wu H, Niu H, Shao A et al (2015) Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar Drugs 13:5750–5766. https://doi.org/10.3390/MD13095750
Popova MP Caffeic acid phenethyl ester (CAPE)-Natural sources, analytical procedures and synthetic approaches. https://doi.org/10.7546/CRABS.2018.09.01
Manochkumar J, Doss CGP, El-Seedi HR et al (2021) The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine 91. https://doi.org/10.1016/J.PHYMED.2021.153676
Murtaza G, Karim S, Akram MR et al (2014) Caffeic acid phenethyl ester and therapeutic potentials. Biomed Res Int 2014. https://doi.org/10.1155/2014/145342
Wan T, Wang Z, Luo Y et al (2019) FA-97, a new synthetic caffeic acid phenethyl ester derivative, protects against oxidative stress-mediated neuronal cell apoptosis and scopolamine-induced cognitive impairment by activating Nrf2/HO-1 signaling. Oxid Med Cell Longev 2019. https://doi.org/10.1155/2019/8239642
Zhang J, Li CY, Xu MJ et al (2012) Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J Ethnopharmacol 144:195–200. https://doi.org/10.1016/J.JEP.2012.09.005
Lee NH, Ho JW (2008) Celastrol and terpenes as anti-infective agents. Antiinfect Agents Med Chem 7:97–100. https://doi.org/10.2174/187152108783954632
Cleren C, Calingasan NY, Chen J, Beal MF (2005) Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem 94:995–1004. https://doi.org/10.1111/j.1471-4159.2005.03253.x
Kulkarni NP, Vaidya B, Narula AS, Sharma SS (2021) Neuroprotective potential of caffeic acid phenethyl ester (CAPE) in CNS disorders: mechanistic and therapeutic insights. Curr Neuropharmacol 19:1401–1415. https://doi.org/10.2174/1570159X19666210608165509
Chow AM, Brown IR (2007) Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones 12:237–244. https://doi.org/10.1379/CSC-269.1
Cleren C, Calingasan NY, Chen J, Beal MF (2005) Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. 94:995–1004. https://doi.org/10.1111/J.1471-4159.2005.03253.X
Duennwald ML (2015) Cellular stress responses in protein misfolding diseases. Futur Sci OA 1. https://doi.org/10.4155/FSO.15.42
Zhang YQ, Sarge KD (2007) Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. 85:1421–1428
Zhang C, Wang R, Liu Z et al (2019) The plant triterpenoid celastrol blocks PINK1-dependent mitophagy by disrupting PINK1’s association with the mitochondrial protein TOM20. J Biol Chem 294:7472–7487. https://doi.org/10.1074/JBC.RA118.006506
Lyu M, Yang C (2021) Neuroprotective effects of celastrol in neurodegenerative diseases-unscramble its major mechanisms of action and targets Platelet-tumor communication View project TCM Attenuates TMAO-Induced Thrombosis via Remodeling of the Gut Microbiota View project. https://doi.org/10.14336/AD.2021.1115
Oglah MK, Fakri Mustafa Y, Kahtan Bashir M et al (2020) Curcumin and its derivatives: a review of their biological activities. Syst Rev Pharm 11:2020. https://doi.org/10.5530/srp.2020.3.60
Egan ME (2004) Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304:600–602. https://doi.org/10.1126/science.1093941
Prakash P, Misra A, Surin WR et al (2011) Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb Res 127:111–118. https://doi.org/10.1016/J.THROMRES.2010.11.007
Sandur SK, Ichikawa H, Pandey MK et al (2007) Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 43:568–580. https://doi.org/10.1016/J.FREERADBIOMED.2007.05.009
Afshariani R, Farhadi P, Ghaffarpasand F, Roozbeh J (2014) Effectiveness of topical curcumin for treatment of mastitis in breastfeeding women: a randomized, double-blind, placebo-controlled clinical trial. Oman Med J 29:330–334. https://doi.org/10.5001/OMJ.2014.89
Talebi M, Kakouri E, Talebi M et al (2021) Nutraceuticals-based therapeutic approach: recent advances to combat pathogenesis of Alzheimer’s disease. Expert Rev Neurother 21:625–642. https://doi.org/10.1080/14737175.2021.1923479
Verma M, Sharma A, Naidu S et al (2012) Curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II complex. PLoS One 7. https://doi.org/10.1371/JOURNAL.PONE.0042923
Elifani F, Amico E, Pepe G, Capocci L, Castaldo S, Rosa P, Montano E, Pollice A (2019) Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington’s disease. Hum Mol Genet. https://doi.org/10.1093/hmg/ddz247
Elifani F, Crispi S, Filosa S et al (2016) L9 Curcumin: a natural compound to counteract the pathology of huntington’s disease? J Neurol Neurosurg Psychiatry 87:A93–A93. https://doi.org/10.1136/JNNP-2016-314597.264
Khatri DK, Juvekar AR (2016) Kinetics of inhibition of monoamine oxidase using curcumin and ellagic acid. Pharmacogn Mag 12(Suppl 2):S116–20. https://doi.org/10.4103/0973-1296.182168
Singh S, Jamwal S, Kumar P (2015) Piperine enhances the protective effect of curcumin against 3-NP induced neurotoxicity: possible neurotransmitters modulation mechanism. Neurochem Res 40:1758–1766. https://doi.org/10.1007/S11064-015-1658-2
Ma X, Tan C, Zhu D et al (2007) Huperzine A from Huperzia species—an ethnopharmacolgical review. J Ethnopharmacol 113:15–34. https://doi.org/10.1016/J.JEP.2007.05.030
Banji OJF, Banji D, Ch K (2014) Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem Toxicol 74:51–59. https://doi.org/10.1016/J.FCT.2014.08.020
Ratia M, Giménez-Llort L, Camps P et al (2013) Huprine X and huperzine A improve cognition and regulate some neurochemical processes related with Alzheimer’s disease in triple transgenic mice (3xTg-AD). Neurodegener Dis 11:129–140. https://doi.org/10.1159/000336427
Qian ZM, Ke Y (2014) Huperzine A: is it an effective disease-modifying drug for Alzheimer’s disease? Front Aging Neurosci 6. https://doi.org/10.3389/FNAGI.2014.00216
Wang R, Yan H, Tang XC (2006) Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine1. Acta Pharmacol Sin 27:1–26. https://doi.org/10.1111/J.1745-7254.2006.00255.X
Bai D (2007) Development of huperzine A and B for treatment of Alzheimer’s disease. Pure Appl Chem 79:469–479. https://doi.org/10.1351/PAC200779040469/MACHINEREADABLECITATION/RIS
Yang G, Wang Y, Tian J, Liu JP (2013) Huperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One 8. https://doi.org/10.1371/JOURNAL.PONE.0074916
Hao Z, Liu M, Liu Z, Lv DH (2009) Huperzine A for vascular dementia. Cochrane database Syst Rev. https://doi.org/10.1002/14651858.CD007365.PUB2
Peavy GM, Jacobson MW, Goldstein JL et al (2010) Cognitive and functional decline in Huntington’s disease: dementia criteria revisited. Mov Disord 25:1163. https://doi.org/10.1002/MDS.22953
Vattakatuchery Jj, Kurien R (2013) Acetylcholinesterase inhibitors in cognitive impairment in Huntington’s disease: a brief review. World J psychiatry 3:62. https://doi.org/10.5498/WJP.V3.I3.62
. Farnsworth NR: The pharmacognosy of the periwinkles:... - Google Scholar. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=.+Farnsworth+NR%3A+The+pharmacognosy+of+the+periwinkles%3A+Vinca+and+Catharanthus.+Lloydia.1961%3B+24+%283%29%3A+105-138.+&btnG=. Accessed 29 Jun 2022
Venkatesan R, Ji E, Kim SY (2015) Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. Biomed Res Int 2015. https://doi.org/10.1155/2015/814068
Varma S, Karwe MV, Lee T-C (2010) Effect of high hydrostatic pressure processing on lycopene isomers. Int J Food Eng 6. https://doi.org/10.2202/1556-3758.1752
Schierle J, Bretzel W, Bühler I et al (1997) Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem 59:459–465. https://doi.org/10.1016/S0308-8146(96)00177-X
Singh P, Goyal GK (2008) Dietary lycopene: its properties and anticarcinogenic effects. Compr Rev food Sci food Saf 7:255–270. https://doi.org/10.1111/J.1541-4337.2008.00044.X
Jain D, Gangshettiwar A (2014) Combination of lycopene, quercetin and poloxamer 188 alleviates anxiety and depression in 3-nitropropionic acid-induced Huntington’s disease in rats. J Intercult Ethnopharmacol 3:186. https://doi.org/10.5455/JICE.20140903012921
Erbakan K, Doğanoğlu A, Erbaş O (2021) Effects of lycopene on neurodegenerative diseases. J Exp Basic Med Sci 2:50–61. https://doi.org/10.5606/jebms.2021.75638
Chen D, Huang C, Chen Z (2019) A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed Pharmacother 111:791–801. https://doi.org/10.1016/J.BIOPHA.2018.12.151
Kumar P, Kalonia H, Kumar A (2010) P1–044: Protective effect of lycopene against 3-nitropropionic acid-induced Huntington’s-like symptoms in rats. Alzheimer’s Dement 6:S186–S186. https://doi.org/10.1016/J.JALZ.2010.05.591
Kumar P, Kalonia H, Kumar A (2009) Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life Sci 85:711–718. https://doi.org/10.1016/J.LFS.2009.10.001
Sandhir R, Mehrotra A, Kamboj SS (2010) Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. 57:579–587. https://doi.org/10.1016/J.NEUINT.2010.07.005
Il BB, Xu H, Igarashi S et al (2005) p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron 47:29–41. https://doi.org/10.1016/J.NEURON.2005.06.005
Jang M, Cho IH (2015) Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-κB pathways. Mol Neurobiol 53(4):2619–2635. https://doi.org/10.1007/S12035-015-9230-2
Manzoor MF, Ahmad N (2000) Food based phytochemical luteolin their derivatives, sources and medicinal benefits. https://doi.org/10.22573/spg.ijals.017.s12200084
Manzoor MF, Ahmad N, Ahmed Z et al (2019) Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J Food Biochem 43. https://doi.org/10.1111/JFBC.12974
Hu W, Wang H, Liu Z et al (2017) Neuroprotective effects of lycopene in spinal cord injury in rats via antioxidative and anti-apoptotic pathway. Neurosci Lett 642:107–112. https://doi.org/10.1016/J.NEULET.2017.02.004
Michels G, Mohamed GA, Weber N et al (2006) Effects of methylated derivatives of Luteolin isolated from Cyperus alopecuroides in rat H4IIE hepatoma cells*. Basic Clin Pharmacol Toxicol 98:168–172. https://doi.org/10.1111/J.1742-7843.2006.PTO_300.X
Seelinger G, Merfort I, Wölfle U, Schempp CM (2008) Anti-carcinogenic effects of the flavonoid luteolin. Molecules 13:2628–2651. https://doi.org/10.3390/MOLECULES13102628
Cheng HY, Hsieh MT, Tsai FS et al (2010) Neuroprotective effect of luteolin on amyloid beta protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother Res 24 Suppl 1: https://doi.org/10.1002/PTR.2940
Choi S-M, Kim BC, Cho Y-H et al (2014) Effects of flavonoid compounds on β-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med J 50:45. https://doi.org/10.4068/CMJ.2014.50.2.45
Oliveira AM, Cardoso SM, Ribeiro M, et al (2015) Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington’s disease mouse striatal cells. 91:1–12. https://doi.org/10.1016/J.NEUINT.2015.10.004
Riccio BVF, Spósito L, Carvalho GC et al (2020) Resveratrol isoforms and conjugates: a review from biosynthesis in plants to elimination from the human body. Arch Pharm (Weinheim) 353:2000146. https://doi.org/10.1002/ARDP.202000146
Khan H, Ullah H, Tundis R et al (2020) Dietary flavonoids in the management of Huntington’s disease: mechanism and clinical perspective. eFood 1:38–52. https://doi.org/10.2991/EFOOD.K.200203.001
Hasan Siddique Y, Rahul, Varshney H et al (2021) Effect of luteolin on the transgenic Drosophila model of Huntington’s disease. Comput Toxicol 17:100148. https://doi.org/10.1016/J.COMTOX.2020.100148
Liu Y, Liu Y, Chen H et al (2015) Synthetic resveratrol derivatives and their biological activities: a review. Open J Med Chem 05:97–105. https://doi.org/10.4236/OJMC.2015.54006
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 1. https://doi.org/10.1038/S41698-017-0038-6
Trela BC, Waterhouse AL (1996) Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem 44:1253–1257. https://doi.org/10.1021/JF9504576
Gambini J, Inglés M, Olaso G et al (2015) Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015. https://doi.org/10.1155/2015/837042
Kuršvietienė L, Stanevičienė I, Mongirdienė A, Bernatonienė J (2016) Multiplicity of effects and health benefits of resveratrol. Medicina (Kaunas) 52:148–155. https://doi.org/10.1016/J.MEDICI.2016.03.003
Pasinetti GM, Wang J, Marambaud P et al (2011) Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp Neurol 232:1–6. https://doi.org/10.1016/J.EXPNEUROL.2011.08.014
Parker JA, Arango M, Abderrahmane S et al (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37:349–350. https://doi.org/10.1038/NG1534
Maher P, Dargusch R, Bodai L et al (2011) ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet 20:261–270. https://doi.org/10.1093/HMG/DDQ460
Vale AP, Santos J, Brito NV et al (2015) Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 115:252–260. https://doi.org/10.1016/j.phytochem.2015.02.004
Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P (2015) Sulforaphane bioavailability from glucoraphanin-rich broccoli: control by active endogenous myrosinase. PLOS ONE 10:e0140963. https://doi.org/10.1371/journal.pone.0140963
Ho DJ, Calingasan NY, Wille E et al (2010) Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol 225:74–84. https://doi.org/10.1016/J.EXPNEUROL.2010.05.006
Vermeulen M, Klöpping-Ketelaars IWAA, Van Den Berg R, Vaes WHJ (2008) Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem 56:10505–10509. https://doi.org/10.1021/JF801989E
Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW (2017) KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 69:257–269. https://doi.org/10.1016/J.TIFS.2017.02.002
Li Q, Fadoul G, Ikonomovic M, Yang T, Zhang F (2022) Sulforaphane promotes white matter plasticity and improves long-term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic Biol Med 193(Pt 1):292–303. https://doi.org/10.1016/j.freeradbiomed.2022.10.001
Kim JK, Park SU (2016) Current potential health benefits of sulforaphane. EXCLI J 15:571–577. https://doi.org/10.17179/EXCLI2016-485
Liu Y, Hettinger CL, Zhang D, Rezvani K, Wang X, Wang H (2014) Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington’s disease. J Neurochem 129:539–547. https://doi.org/10.1111/jnc.12647
Subedi L, Cho K, Park YU et al (2019) Sulforaphane-enriched broccoli sprouts pretreated by pulsed electric fields reduces neuroinflammation and ameliorates scopolamine-induced amnesia in mouse brain through its antioxidant ability via Nrf2-HO-1 activation. Oxid Med Cell Longev 2019. https://doi.org/10.1155/2019/3549274
Ulusoy A, Di Monte DA (2012) α-Synuclein elevation in human neurodegenerative diseases: experimental, pathogenetic, and therapeutic implications. Mol Neurobiol 47(2):484–494. https://doi.org/10.1007/S12035-012-8329-Y
Cai X, Seitl I, Mu W et al (2018) Biotechnical production of trehalose through the trehalose synthase pathway: current status and future prospects. Appl Microbiol Biotechnol 102(7):2965–2976. https://doi.org/10.1007/S00253-018-8814-Y
Tanaka M, Machida Y, Niu S et al (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10:148–154. https://doi.org/10.1038/NM985
Brokowska J, Hać A, Węgrzyn G, Herman-Antosiewicz A (2016) L12 Sulforaphane reduces the level of exogenous mutated huntingtin protein in normal human fibroblasts. J Neurol Neurosurg Psychiatry 87:A94–A94. https://doi.org/10.1136/JNNP-2016-314597.267
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13. https://doi.org/10.1093/GLYCOB/CWG047
Ohtake S, Wang YJ (2011) Trehalose: current use and future applications. J Pharm Sci 100:2020–2053. https://doi.org/10.1002/JPS.22458
Khalifeh M, Barreto GE, Sahebkar A (2019) Trehalose as a promising therapeutic candidate for the treatment of Parkinson’s disease. Br J Pharmacol 176:1173–1189. https://doi.org/10.1111/BPH.14623
Fernandez-Estevez MA, Casarejos MJ, Sendon JL et al (2014) Trehalose reverses cell malfunction in fibroblasts from normal and Huntington’s disease patients caused by proteosome inhibition. PLoS One 9:e90202. https://doi.org/10.1371/JOURNAL.PONE.0090202
Sgarbossa A (2012) Natural biomolecules and protein aggregation: emerging strategies against amyloidogenesis. Int J Mol Sci 13:17121–17137. https://doi.org/10.3390/IJMS131217121
Zeng Y, Guo W, Xu G et al (2016) Xyloketal-derived small molecules show protective effect by decreasing mutant Huntingtin protein aggregates in Caenorhabditis elegans model of Huntington’s disease. Drug Des Devel Ther 10:1443–1451. https://doi.org/10.2147/DDDT.S94666
Wong VKW, Wu AG, Wang JR et al (2015) Neferine attenuates the protein level and toxicity of mutant huntingtin in PC-12 cells via induction of autophagy. Molecules 20:3496–3514. https://doi.org/10.3390/MOLECULES20033496
Wu AG, Wong VKW, Xu SW et al (2013) Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant α-synuclein and huntingtin in PC-12 cells. Int J Mol Sci 14:22618–22641. https://doi.org/10.3390/IJMS141122618
Walter GM, Raveh A, Mok SA et al (2014) High-throughput screen of natural product extracts in a yeast model of polyglutamine proteotoxicity. Chem Biol Drug Des 83:440–449. https://doi.org/10.1111/CBDD.12259
Sun A, Xu X, Lin J et al (2015) Neuroprotection by saponins. Phytother Res 29:187–200. https://doi.org/10.1002/PTR.5246
Munoz-Sanjuan I, Bates GP (2011) The importance of integrating basic and clinical research toward the development of new therapies for Huntington disease. J Clin Invest 121:476–483. https://doi.org/10.1172/JCI45364
Bonelli R, Wenning G (2006) Pharmacological management of Huntington’s disease: an evidence-based review. Curr Pharm Des 12:2701–2720. https://doi.org/10.2174/138161206777698693
Braun MM, Farag-El-Massah S, Xu K, Coté TR (2010) Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov 9:519–522. https://doi.org/10.1038/NRD3160
Woodcock J (2012) The future of orphan drug development. Clin Pharmacol Ther 92:146–148. https://doi.org/10.1038/CLPT.2012.89
Angus D, Herd C, Stone C et al (2015) Safety, tolerability, and efficacy of PBT2 in Huntington’s disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol 14:39–47. https://doi.org/10.1016/S1474-4422(14)70262-5
De Yebenes JG, Landwehrmeyer B, Squitieri F et al (2011) Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol 10:1049–1057. https://doi.org/10.1016/S1474-4422(11)70233-2
Karl K, McGarry A, McDermott MP et al (2013) A randomized, double-blind, placebo-controlled trial of pridopidine in Huntington’s disease. Mov Disord 28:1407–1415. https://doi.org/10.1002/MDS.25362
Levin SW, Baker EH, Zein WM, Zhang Z, Quezado ZMN, Miao N, Gropman A, Griffin KJ et al (2014) Oral cysteamine bitartrate and N-acetylcysteine for patients with infantile neuronal ceroid lipofuscinosis: a pilot study. Lancet Neurol 13:777–787. https://doi.org/10.1016/s1474-4422(14)70142-5
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL et al (2009) Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. The Lancet Neurology 8:791–801. https://doi.org/10.1016/s1474-4422(09)70170-x
Hersch SM, Schifitto G, Oakes D et al (2017) The CREST-E study of creatine for Huntington disease: a randomized controlled trial. Neurology 89:594–601. https://doi.org/10.1212/WNL.0000000000004209
Rodrigues FB, Duarte GS, Costa J et al (2017) Tetrabenazine versus deutetrabenazine for Huntington’s disease: twins or distant cousins? Mov Disord Clin Pract 4:582–585. https://doi.org/10.1002/MDC3.12483
Claassen DO, Carroll B, De Boer LM, Wu E, Ayyagari R, Gandhi S, Stamler D (2017) Indirect tolerability comparison of Deutetrabenazine and Tetrabenazine for Huntington disease. J Clin Mov Disord 41(4):1–11. https://doi.org/10.1186/S40734-017-0051-5
Rodrigues FB, Duarte GS, Costa J, Ferreira JJ, Wild JJ (2017) Meta-research metrics matter: letter regarding article “indirect tolerability comparison of Deutetrabenazine and Tetrabenazine for Huntington disease.” J Clin Mov Disord 41(4):1–3. https://doi.org/10.1186/S40734-017-0067-X
Choudhary S, Kumar P, Malik J (2013) Plants and phytochemicals for Huntington’s disease. Pharmacogn Rev 7:81–91. https://doi.org/10.4103/0973-7847.120505
Acknowledgements
This paper was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Author information
Authors and Affiliations
Contributions
MRL, MHJ, GKT: conceptualization, formal analysis, writing—original draft; SA, PSD, AR, BV: investigation, writing—review and editing; MMR, TA, MA, HAH: resources, supervision, writing— review and editing; MT: resources, writing—review and editing; BAR, BV: conceptualization, resources, supervision, writing— original draft.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Islam, M.R., Jony, M.H., Thufa, G.K. et al. A clinical study and future prospects for bioactive compounds and semi-synthetic molecules in the therapies for Huntington's disease. Mol Neurobiol 61, 1237–1270 (2024). https://doi.org/10.1007/s12035-023-03604-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03604-4