Abstract
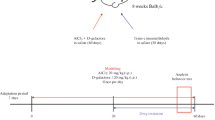
Mitochondrial dysfunction plays a fundamental role in the pathogenesis of cognitive deficit. Rutaecarpine (Rut) is a natural alkaloid with anti-inflammatory and antioxidant properties. This study explored whether Rut treatment could enhance cognitive function by improving mitochondrial function and examined the potential mechanisms underlying this ameliorative effect. We used the Morris water maze and Y-maze tests to evaluate the behavioral effects of Rut in a mouse model of cognitive impairment induced by subcutaneous injection of D-galactose (D-gal). Furthermore, we assessed the effects of Rut on mitochondrial function using cell viability assays, flow cytometry, western blotting, biochemical analysis, and immunochemical techniques in vivo and in vitro. The results indicated Rut treatment attenuated cognitive deficits and mitochondrial dysfunction in the mouse model. Similarly, it maintained the balance of mitochondrial dynamics in neurocytes and reduced oxidative stress and mitochondrial apoptosis in the HT22 cell model. Moreover, we found that these protective effects were dependent on the activation of the AMP-activated protein kinase/proliferator-activated receptor gamma coactivator 1-alpha (AMPK/PGC1α) signaling pathway. Our data indicate that Rut treatment are sensitive to reversal cognitive deficits and mitochondrial dysfunction induced by D-gal; this suggests that Rut is a promising mitochondria-targeted therapeutic agent for treating cognitive impairment.







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- D-gal:
-
D-galactose
- Rut:
-
Rutaecarpine
- AMPK:
-
Adenosine 5′-monophosphate-activated protein kinase
- PGC1α:
-
Peroxisome proliferator-activated receptor-γ coactivator 1α
- Nrf1:
-
Nuclear respiratory factor 1
- TFAM:
-
Mitochondrial transcription factor A
- UCP2:
-
Uncoupling protein 2
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH:
-
Reduced glutathione
- Drp1:
-
Dynamic-related protein 1
- Mfn-2:
-
Mitofusin 2
- CC3:
-
cleaved Caspase-3
- Cytochrome C:
-
Cyt-c
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ROS:
-
Reactive oxygen species
- TUNEL:
-
Terminal deoxynucleotidyl transferase-dUTP nick end labeling
- MMP:
-
Mitochondrial membrane potential
- WT:
-
Wild type
- MWM:
-
Morris Water Maze
- TEM:
-
Transmission electron microscopy
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Erkkinen MG, Kim MO, Geschwind MD (2018) Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol 10(4). https://doi.org/10.1101/cshperspect.a033118
Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, Li Y, Li Y et al (2020) Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5(12):e661–e671. https://doi.org/10.1016/s2468-2667(20)30185-7
2022 Alzheimer's disease facts and figures (2022) Alzheimers Dement 18 (4):700–789. https://doi.org/10.1002/alz.12638
Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q et al (2020) Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol 19(1):81–92. https://doi.org/10.1016/s1474-4422(19)30290-x
Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, Zhao L, Jin H et al (2018) The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement 14(4):483–491. https://doi.org/10.1016/j.jalz.2017.12.006
Sharma C, Kim S, Nam Y, Jung UJ, Kim SR (2021) Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094850
Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr et al (2019) Understanding disease progression and improving Alzheimer’s disease clinical trials: recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 15(1):106–152. https://doi.org/10.1016/j.jalz.2018.08.005
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 8:1219–1231. https://doi.org/10.1016/j.bbadis.2013.09.010
Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S (2020) PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev 2020:1452696. https://doi.org/10.1155/2020/1452696
Al-Damry NT, Attia HA, Al-Rasheed NM, Al-Rasheed NM, Mohamad RA, Al-Amin MA, Dizmiri N, Atteya M (2018) Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3β and p38α/MAPK in a rat model of diabetic cardiomyopathy. Biomed Pharmacother 107:347–358. https://doi.org/10.1016/j.biopha.2018.07.126
Wang Y, Zhang T, Zhao H, Qi C, Ji X, Yan H, Cui R, Zhang G et al (2021) Pentoxifylline enhances antioxidative capability and promotes mitochondrial biogenesis in D-galactose-induced aging mice by increasing Nrf2 and PGC-1α through the cAMP-CREB pathway. Oxid Med Cell Longev 2021:6695613. https://doi.org/10.1155/2021/6695613
Son JK, Chang HW, Jahng Y (2015) Progress in studies on rutaecarpine. II.--Synthesis and Structure-Biological Activity Relationships. Molecules 20(6):10800–10821. https://doi.org/10.3390/molecules200610800
Zhang Y, Yan T, Sun D, Xie C, Wang T, Liu X, Wang J, Wang Q et al (2020) Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis. Free Radic Biol Med 148:33–41. https://doi.org/10.1016/j.freeradbiomed.2019.12.012
Lee GH, Kim CY, Zheng C, Jin SW, Kim JY, Lee SY, Kim MY, Han EH et al (2021) Rutaecarpine increases nitric oxide synthesis via eNOS phosphorylation by TRPV1-dependent CaMKII and CaMKKβ/AMPK signaling pathway in human endothelial cells. Int J Mol Sci 22(17). https://doi.org/10.3390/ijms22179407
Sahoo AK, Dandapat J, Dash UC, Kanhar S (2018) Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J Ethnopharmacol 215:42–73. https://doi.org/10.1016/j.jep.2017.12.015
Zhao B, Wang Y, Liu R, Jia XL, Hu N, An XW, Zheng CG, Chen C et al (2021) Rutaecarpine ameliorated high sucrose-induced Alzheimer’s disease like pathological and cognitive impairments in mice. Rejuvenation Res 24(3):181–190. https://doi.org/10.1089/rej.2020.2349
Zhang YT, Li Z, Zhang K, Zhang HY, He ZH, Xia Q, Zhao JH, Feng NP (2017) Co-delivery of evodiamine and rutaecarpine in a microemulsion-based hyaluronic acid hydrogel for enhanced analgesic effects on mouse pain models. Int J Pharm 528(1-2):100–106. https://doi.org/10.1016/j.ijpharm.2017.05.064
Li Z, Yang M, Peng Y, Gao M, Yang B (2019) Rutaecarpine ameliorated sepsis-induced peritoneal resident macrophages apoptosis and inflammation responses. Life Sci 228:11–20. https://doi.org/10.1016/j.lfs.2019.01.038
Dai Z, Xiao J, Liu SY, Cui L, Hu GY, Jiang DJ (2008) Rutaecarpine inhibits hypoxia/reoxygenation-induced apoptosis in rat hippocampal neurons. Neuropharmacology 55(8):1307–1312. https://doi.org/10.1016/j.neuropharm.2008.08.030
Liang CJ, Li JH, Zhang Z, Zhang JY, Liu SQ, Yang J (2018) Suppression of MIF-induced neuronal apoptosis may underlie the therapeutic effects of effective components of Fufang Danshen in the treatment of Alzheimer’s disease. Acta Pharmacol Sin 39(9):1421–1438. https://doi.org/10.1038/aps.2017.210
Mansour HM, Fawzy HM, El-Khatib AS, Khattab MM (2021) Inhibition of mitochondrial pyruvate carrier 1 by lapatinib ditosylate mitigates Alzheimer’s-like disease in D-galactose/ovariectomized rats. Neurochem Int 150:105178. https://doi.org/10.1016/j.neuint.2021.105178
Suryavanshi PS, Ugale RR, Yilmazer-Hanke D, Stairs DJ, Dravid SM (2014) GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br J Pharmacol 171(3):799–809. https://doi.org/10.1111/bph.12518
Jia N, Sun Q, Su Q, Dang S, Chen G (2016) Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1α pathway. Redox Biol 10:179–190. https://doi.org/10.1016/j.redox.2016.10.004
Chuang JI, Pan IL, Hsieh CY, Huang CY, Chen PC, Shin JW (2016) Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res 61(2):230–240. https://doi.org/10.1111/jpi.12343
Tian Y, Zou B, Yang L, Xu SF, Yang J, Yao P, Li CM (2011) High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by D-galactose. Food Chem Toxicol 49(8):1728–1736. https://doi.org/10.1016/j.fct.2011.04.018
Wang G, Wang Y, Yang Q, Xu C, Zheng Y, Wang L, Wu J, Zeng M et al (2022) Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis 13(1):29. https://doi.org/10.1038/s41419-021-04478-x
Andrabi SS, Ali M, Tabassum H, Parveen S, Parvez S (2019) Pramipexole prevents ischemic cell death via mitochondrial pathways in ischemic stroke. Dis Model Mech 12(8). https://doi.org/10.1242/dmm.033860
Ding M, Feng N, Tang D, Feng J, Li Z, Jia M, Liu Z, Gu X et al (2018) Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J Pineal Res 65(2):e12491. https://doi.org/10.1111/jpi.12491
Aslam M, Ladilov Y (2022) Emerging role of cAMP/AMPK signaling. Cells 11(2). https://doi.org/10.3390/cells11020308
Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L, Reiter RJ, Qiao S et al (2018) AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med 129:59–72. https://doi.org/10.1016/j.freeradbiomed.2018.08.032
Ali T, Badshah H, Kim TH, Kim MO (2015) Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J Pineal Res 58(1):71–85. https://doi.org/10.1111/jpi.12194
Kantar Gok D, Ozturk N, Er H, Aslan M, Demir N, Derin N, Agar A, Yargicoglu P (2015) Effects of rosmarinic acid on cognitive and biochemical alterations in ovariectomized rats treated with D-galactose. Folia Histochem Cytobiol 53(4):283–293. https://doi.org/10.5603/fhc.a2015.0034
Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2018) Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol 101:13–36. https://doi.org/10.1016/j.exger.2017.10.029
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J (2006) Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 83(8):1584–1590. https://doi.org/10.1002/jnr.20845
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583-019-0132-6
Wang J, Fröhlich H, Torres FB, Silva RL, Poschet G, Agarwal A, Rappold GA (2022) Mitochondrial dysfunction and oxidative stress contribute to cognitive and motor impairment in FOXP1 syndrome. Proc Natl Acad Sci U S A 119(8). https://doi.org/10.1073/pnas.2112852119
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS et al (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947. https://doi.org/10.1016/j.redox.2021.101947
Lotfi M, Kazemi S, Ebrahimpour A, Pourabdolhossein F, Satarian L, Eghbali A, Moghadamnia AA (2022) Thymoquinone improved nonylphenol-induced memory deficit and neurotoxicity through its antioxidant and neuroprotective effects. Mol Neurobiol 59(6):3600–3616. https://doi.org/10.1007/s12035-022-02807-5
Nickel A, Kohlhaas M, Maack C (2014) Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73:26–33. https://doi.org/10.1016/j.yjmcc.2014.03.011
Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ (2015) Redox homeostasis and mitochondrial dynamics. Cell Metab 22(2):207–218. https://doi.org/10.1016/j.cmet.2015.06.006
Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF (2010) Purple sweet potato color alleviates D-galactose-induced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibiting cytochrome C-mediated apoptosis. Brain Pathol 20(3):598–612. https://doi.org/10.1111/j.1750-3639.2009.00339.x
Meng L, Xin G, Li B, Li D, Sun X, Yan T, Li L, Shi L et al (2018) Anthocyanins extracted from aronia melanocarpa protect SH-SY5Y cells against amyloid-beta (1-42)-induced apoptosis by regulating Ca(2+) homeostasis and inhibiting mitochondrial dysfunction. J Agric Food Chem 66(49):12967–12977. https://doi.org/10.1021/acs.jafc.8b05404
Zhao W, Xu Z, Cao J, Fu Q, Wu Y, Zhang X, Long Y, Zhang X et al (2019) Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation 16(1):230. https://doi.org/10.1186/s12974-019-1627-9
Wang H, Liu B, Yin X, Guo L, Jiang W, Bi H, Guo D (2018) Excessive zinc chloride induces murine photoreceptor cell death via reactive oxygen species and mitochondrial signaling pathway. J Inorg Biochem 187:25–32. https://doi.org/10.1016/j.jinorgbio.2018.07.004
Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y et al (2021) SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev 2021:9265016. https://doi.org/10.1155/2021/9265016
Wang W, Yin J, Ma X, Zhao F, Siedlak SL, Wang Z, Torres S, Fujioka H et al (2017) Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum Mol Genet 26(21):4118–4131. https://doi.org/10.1093/hmg/ddx299
Dhapola R, Sarma P, Medhi B, Prakash A, Reddy DH (2022) Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer’s disease. Mol Neurobiol 59(1):535–555. https://doi.org/10.1007/s12035-021-02612-6
Hu C, Huang Y, Li L (2017) Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci 18(1). https://doi.org/10.3390/ijms18010144
Liu D, Li J, Rong X, Li J, Peng Y, Shen Q (2022) Cdk5 promotes mitochondrial fission via Drp1 phosphorylation at S616 in chronic ethanol exposure-induced cognitive impairment. Mol Neurobiol 59(12):7075–7094. https://doi.org/10.1007/s12035-022-03008-w
Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen Y, Fu Z (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res 66(2):e12542. https://doi.org/10.1111/jpi.12542
Xu W, Yan J, Ocak U, Lenahan C, Shao A, Tang J, Zhang J, Zhang JH (2021) Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics 11(2):522–539. https://doi.org/10.7150/thno.49426
Fan H, Ding R, Liu W, Zhang X, Li R, Wei B, Su S, Jin F et al (2021) Heat shock protein 22 modulates NRF1/TFAM-dependent mitochondrial biogenesis and DRP1-sparked mitochondrial apoptosis through AMPK-PGC1α signaling pathway to alleviate the early brain injury of subarachnoid hemorrhage in rats. Redox Biol 40:101856. https://doi.org/10.1016/j.redox.2021.101856
Hang W, He B, Chen J, Xia L, Wen B, Liang T, Wang X, Zhang Q et al (2018) Berberine ameliorates high glucose-induced cardiomyocyte injury via AMPK signaling activation to stimulate mitochondrial biogenesis and restore autophagic flux. Front Pharmacol 9:1121. https://doi.org/10.3389/fphar.2018.01121
Hasegawa K, Yasuda T, Shiraishi C, Fujiwara K, Przedborski S, Mochizuki H, Yoshikawa K (2016) Promotion of mitochondrial biogenesis by necdin protects neurons against mitochondrial insults. Nat Commun 7:10943. https://doi.org/10.1038/ncomms10943
Acknowledgements
We wish to thank Dr. Heng Zhang, Dr. Di-yang Lyv, Dr. Wen-ying Liu, Dr. Xue-chu Wang, Dr. Mengmeng Guo, Dr. Tan Zhao, Dr. Yan Li, and Dr. Yue Zhang for their generous assistant with performing experiments and suggestions on revising the manuscript. We wish to thank Bing-qiu Li, Ling-zhi Xu, Wen-wen Li, Ya-na Pang, and Mei-na Quan for their guidance.
Funding
This study was supported by the Key Project of the National Natural Science Foundation of China (U20A20354), Beijing Brain Initiative from Beijing Municipal Science & Technology Commission (Z201100005520016, Z201100005520017), National major R&D projects of China-Scientific technological innovation 2030 (2021ZD0201802), the National Key Scientific Instrument and Equipment Development Project (31627803), and the Key Project of the National Natural Science Foundation of China (81530036).
Author information
Authors and Affiliations
Contributions
Jianping Jia designed the experiments, conceived the study concept, and revised the manuscript. Min Gong designed and performed the experiments, analyzed the data, and wrote the manuscript draft.
Corresponding author
Ethics declarations
Ethics Approval
All animals were treated according to the guidelines for the Care and Use of Laboratory Animals and the experimental procedures were approved by the Animal Welfare Ethics Committee (Approval number: VS212601429).
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 5687 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, M., Jia, J. Rutaecarpine Mitigates Cognitive Impairment by Balancing Mitochondrial Function Through Activation of the AMPK/PGC1α Pathway. Mol Neurobiol 60, 6598–6612 (2023). https://doi.org/10.1007/s12035-023-03505-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03505-6