Abstract
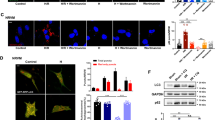
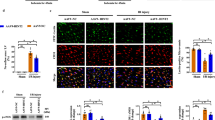
Irisin is a muscle-derived hormone that promotes the survival of motor neurons and enhances muscle size following injury. In this study, we investigated the beneficial effects and mechanism(s) of action of irisin in response to cerebral ischemia–reperfusion injury (CIRI). Right-middle cerebral artery occlusion (MCAO) and hypoxia/reoxygenation (H/R) models were generated in C57BL/6 J mice. Mouse neuronal cell lines (NSC-34) were used to confirm the molecular mechanisms of the protection afforded by irisin in response to CIRI. We found that irisin (250 μg/kg) improved cerebral function and reduced the cerebral infarct volume following CIRI. Irisin also protected neuronal cells against ischemia–reperfusion (I/R) induced apoptosis, assessed via TUNEL, and cleaved Caspase-3 staining. Western blotting of neuronal tissue from irisin treated I/R mice showed lower expression of pro-apoptotic Bax and caspase-9 (P < 0.001 and P < 0.01) and increased levels of the pro-survival protein Bcl-2 (P < 0.01 & P < 0.001 vs. I/R). Irisin also reduced the levels of reactive oxygen species (ROS) characterized through malondialdehyde (MDA) assays. Irisin was found to maintain mitochondrial homeostasis through the suppression of mitochondrial fission-linked dynamin-related protein 1 in CIRI mice (P < 0.01 and P < 0.05 v. I/R cohort). Moreover, mitochondrial fusion–related protein (Mfn2) and Opa1 expression were rescued following irisin treatment (P < 0.001 and P < 0.01 v. I/R cohort). Cell-based assays showed that irisin activates PI3K/AKT/mTOR signaling in the neurons of CIRI mice. Furthermore, the beneficial effects of irisin on NSC-34 cell-survival, mitochondrial function, and ROS generation were reversed by VS-5584, a highly specific PI3K/AKT/mTOR inhibitor. Collectively, these data highlight the ability of irisin to alleviate CIRI in vivo and in vitro. The mechanisms of action of irisin include the attenuation of apoptosis through the prevention of mitochondrial fission and increased mitochondrial fusion and the alleviation of oxidative stress through activation of the PI3K/AKT/mTOR axis. We therefore identify irisin as a much-needed therapeutic for CIRI.





Similar content being viewed by others
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
References
Cerqueira NF, Hussni CA, Yoshida WB (2005) Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras 20:336–343. https://doi.org/10.1590/s0102-86502005000400013
Dong Y, Bao C, Yu J, Liu X (2016) Receptor-interacting protein kinase 3-mediated programmed cell necrosis in rats subjected to focal cerebral ischemia-reperfusion injury. Mol Med Rep 14:728–736. https://doi.org/10.3892/mmr.2016.5311
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 6:524–551. https://doi.org/10.1016/j.redox.2015.08.020
Wang J, Wang P, Li S, Wang S, Li Y, Liang N, Wang M (2014) Mdivi-1 prevents apoptosis induced by ischemia-reperfusion injury in primary hippocampal cells via inhibition of reactive oxygen species-activated mitochondrial pathway. J Stroke Cerebrovasc Dis 23:1491–1499. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.12.021
Liu J, Li J, Yang Y, Wang X, Zhang Z, Zhang L (2014) Neuronal apoptosis in cerebral ischemia/reperfusion area following electrical stimulation of fastigial nucleus. Neural Regen Res 9:727–734. https://doi.org/10.4103/1673-5374.131577
Li K, Ding D, Zhang M (2016) Neuroprotection of osthole against cerebral ischemia/reperfusion injury through an anti-apoptotic pathway in rats. Biol Pharm Bull 39:336–342. https://doi.org/10.1248/bpb.b15-00699
Archer SL (2013) Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med 369:2236–2251. https://doi.org/10.1056/NEJMra1215233
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ (2016) Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351:275–281. https://doi.org/10.1126/science.aab4138
Li Y, Liu X (2018) Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol 233:5589–5597. https://doi.org/10.1002/jcp.26522
Wang L, Kriegstein A (2020) Mitochondria control cortical cell fate after mitosis. Dev Cell 55:120–122. https://doi.org/10.1016/j.devcel.2020.09.028
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA et al (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777
Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, Lee Y, Zhang L et al (2015) Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 87:138–147. https://doi.org/10.1016/j.yjmcc.2015.07.015
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L et al (2019) Corrigendum to “Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress.” Redox Biol 26:101193. https://doi.org/10.1016/j.redox.2019.101193. ([Redox Biol. 20 (2019) 296-306])
Chen K, Xu Z (2017) Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. 9:eaao6298. https://doi.org/10.1126/scitranslmed.aao6298
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38:3032–3039. https://doi.org/10.1161/strokeaha.107.488445
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293. https://doi.org/10.1038/jcbfm.1990.47
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Zhang J, Song S, Pang Q, Zhang R, Zhou L, Liu S, Meng F, Wu Q, Liu C (2015) Serotonin deficiency exacerbates acetaminophen-induced liver toxicity in mice. Sci Rep 5:8098. https://doi.org/10.1038/srep08098
Xu D, Du W, Zhao L, Davey AK, Wang J (2008) The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Med 74:816–821. https://doi.org/10.1055/s-2008-1074557
Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, Menon DK, Pickard JD, Kirkpatrick PJ (2014) The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 85:1343–1353. https://doi.org/10.1136/jnnp-2014-307711
Xu H, Zhang Y, Sun H, Chen S, Wang F (2014) Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS One 9:e97488. https://doi.org/10.1371/journal.pone.0097488
Aydin S, Kuloglu T, Aydin S, Eren MN, Celik A, Yilmaz M, Kalayci M, Sahin B, Gungor O, Gurel A (2014) Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: cardiac muscle produces more irisin than skeletal muscle. Peptides 52:68–73
Wrann C, White J, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin J, Greenberg M, Spiegelman B (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659
Varela-Rodríguez B, Pena-Bello L, Juiz-Valiña AP, Vidal-Bretal B, Cordido F, Sangiao-Alvarellos S (2016) FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Scientific Reports 6:29898
Wang K, Li H, Wang H, Wang JH, Song F, Sun Y (2018) Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediators Inflamm 2018:1–7
Zhang M, Chen P, Chen S, Sun Q, Zeng QC, Chen JY, Liu YX, Cao XH et al (2014) The association of new inflammatory markers with type 2 diabetes mellitus and macrovascular complications: a preliminary study. Eur Rev Med Pharmacol Sci 18:1567–1572
Panati K, Suneetha Y, Narala VR (2016) Irisin/FNDC5–an updated review. Eur Rev Med Pharmacol Sci 20:689–697
Li YZ, Liu XH (2018) Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. Journal of Cellular Physiology 233(8):5589–5597
Tu T, Peng J, Jiang Y (2020) FNDC5/Irisin: a new protagonist in acute brain injury. Stem Cells and Development 29:533–543
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C (2019) Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol 20:296–306
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337:1062–1065. https://doi.org/10.1126/science.1219855
Li J, Qi M, Li C, Shi D, Zhang D, Xie D, Yuan T, Feng J et al (2014) Tom70 serves as a molecular switch to determine pathological cardiac hypertrophy. Cell Res 24:977–993. https://doi.org/10.1038/cr.2014.94
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF (2014) CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 5:3596. https://doi.org/10.1038/ncomms4596
Grohm J, Kim SW, Mamrak U, Tobaben S, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19:1446–1458
Ylabc D, Plabc D, Mcac D, Yzbc D, Jcac D, Mzac D, Ji L, Hdac D, Rcac D, Xpa D (2020) Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biology 34:101503.
Anzell AR, Maizy R, Przyklenk K, Sanderson TH (2018) Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol 55:2547–2564. https://doi.org/10.1007/s12035-017-0503-9
Kameoka S, Adachi Y, Okamoto K, Iijima M, Sesaki H (2018) Phosphatidic acid and cardiolipin coordinate mitochondrial dynamics. Trends Cell Biol 28:67–76. https://doi.org/10.1016/j.tcb.2017.08.011
Sun W, Zeng CR, Yue D, Hu YC (2019) Involvement of mitochondrial dysfunction in hepatotoxicity induced by Ageratina adenophora in mice. J Zhejiang Univ Sci B 20:693–698. https://doi.org/10.1631/jzus.B1800645
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L et al (2019) Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol 20:296–306. https://doi.org/10.1016/j.redox.2018.10.019
Escobar-Henriques M, Anton F (2018) Mechanistic perspective of mitochondrial fusion: tubulation vs. fragmentation. Biochim Biophys Acta 1833:162–75.
Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25:2966–2977
Maciejczyk M, Żebrowska E, Chabowski A (2019) Insulin resistance and oxidative stress in the brain: what's new? Int J Mol Sci 20:874. https://doi.org/10.3390/ijms20040874
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M (2013) Molecular mechanisms of ischemia–reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47:9–23
Yu ZH, Cai M, Xiang J, Zhang ZN, Zhang JS, Song XL, Zhang W, Bao J et al (2016) PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol 181:8–19. https://doi.org/10.1016/j.jep.2016.01.028
Zhang Q, An R, Tian X, Yang M, Li M, Lou J, Xu L, Dong Z (2017) β-Caryophyllene pretreatment alleviates focal cerebral ischemia-reperfusion injury by activating PI3K/Akt signaling pathway. Neurochem Res 42:1459–1469. https://doi.org/10.1007/s11064-017-2202-3
Bockaert J, Marin P (2015) mTOR in brain physiology and pathologies. Physiol Rev 95:1157–1187. https://doi.org/10.1152/physrev.00038.2014
Jiao S, Zhu H, He P, Teng J (2016) Betulinic acid protects against cerebral ischemia/reperfusion injury by activating the PI3K/Akt signaling pathway. Biomed Pharmacother 84:1533–1537. https://doi.org/10.1016/j.biopha.2016.11.028
Zhang M, Xu Y, Jiang L (2019) Irisin attenuates oxidized low-density lipoprotein impaired angiogenesis through AKT/mTOR/S6K1/Nrf2 pathway. J Cell Physiol 234:18951–18962. https://doi.org/10.1002/jcp.28535
Peng Q, Wang X, Wu K, Liu K, Chen X (2017) Irisin attenuates H2O2-induced apoptosis in cardiomyocytes via microRNA-19b/AKT/mTOR signaling pathway. Int J Clin Exp Pathol 10:7707–7717
Funding
This study was supported by the National Natural Science Foundation of China (31870335); the Natural Science Foundation of Gansu Provincial Department of Science and Technology (20JR5RA344); the Gansu Province Health Industry Scientific Research Project (GSWSKY2021-017); the Lanzhou City Chengguan District Science and Technology Project (2021–9-3); and the “Cuiying Technology Innovation” Planning Project of Lanzhou University Second Hospital (CY2021-MS-B01).
Author information
Authors and Affiliations
Contributions
JFL, GS, and ZCZ designed the experiments; LXC, JPZ, JG, JJZ, and QHW performed the experiments; WC, DYC, and ZCZ analyzed the data; JFL and GS prepared the manuscript. All authors read and approved submission of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Research Involving Human Participants and/or Animals
All in vivo protocols were accepted by the Institutional Animal Care and Use Committee of the Ethics Committee of Lanzhou University Second Hospital, according to regulations imposed by the China Council on Animal Care and Use.
Informed Consent
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Jf., Su, G., Chen, Lx. et al. Irisin Attenuates Apoptosis Following Ischemia–Reperfusion Injury Through Improved Mitochondria Dynamics and ROS Suppression Mediated Through the PI3K/Akt/mTOR Axis. Mol Neurobiol 60, 4261–4272 (2023). https://doi.org/10.1007/s12035-023-03336-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03336-5