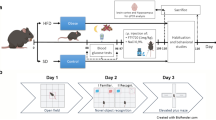
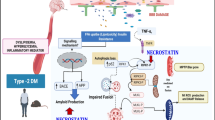
Abstract
Sphingosine receptors (S1PRs) are implicated in the progression of neurodegenerative diseases and metabolic disorders like obesity and type 2 diabetes (T2D). The link between S1PRs and cognition in type 2 diabetes, as well as the mechanisms that underpin it, are yet unknown. Neuroinflammation is the common pathology shared among T2D and cognitive impairment. However, the interplay between the M1 and M2 polarization state of microglia, a primary driver of neuroinflammation, could be the driving factor for impaired learning and memory in diabetes. In the present study, we investigated the effects of fingolimod (S1PR1 modulator) on cognition in high-fat diet and streptozotocin-induced diabetic mice. We further assessed the potential pathways linking microglial polarization and cognition in T2D. Fingolimod (0.5 mg/kg and 1 mg/kg) improved M2 polarization and synaptic plasticity while ameliorating cognitive decline and neuroinflammation. Sphingolipid dysregulation was mimicked in vitro using palmitate in BV2 cells, followed by conditioned media exposure to Neuro2A cells. Mechanistically, type 2 diabetes induced microglial activation, priming microglia towards the M1 phenotype. In the hippocampus and cortex of type 2 diabetic mice, there was a substantial drop in pSTAT3, which was reversed by fingolimod. This protective effect of fingolimod on microglial M2 polarization was primarily suppressed by selective jmjd3 blockade in vitro using GSK-J4, revealing that jmjd3 was involved downstream of STAT3 in the fingolimod-enabled shift of microglia from M1 to M2 polarization state. This study suggested that fingolimod might effectively improve cognition in type 2 diabetes by promoting M2 polarization.















Similar content being viewed by others
Data Availability
The data that support the findings of this study is available in the manuscript as well as supplementary information file. In addition, data can be available from the corresponding author upon reasonable request.
References
Rom S, Zuluaga-Ramirez V, Gajghate S et al (2019) Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) Type 1 and type 2 mouse models. Mol Neurobiol 56:1883–1896. https://doi.org/10.1007/s12035-018-1195-5
McMillan JM, Mele BS, Hogan DB, Leung AA (2018) Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res Care 6:e000563. https://doi.org/10.1136/bmjdrc-2018-000563
Mallorquí-Bagué N, Lozano-Madrid M, Toledo E et al (2018) Type 2 diabetes and cognitive impairment in an older population with overweight or obesity and metabolic syndrome: baseline cross-sectional analysis of the PREDIMED-plus study. Sci Rep 8:16128. https://doi.org/10.1038/s41598-018-33843-8
Biessels GJ, Staekenborg S, Brunner E et al (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74. https://doi.org/10.1016/S1474-4422(05)70284-2
Marseglia A, Darin-Mattsson A, Skoog J et al (2021) Metabolic Syndrome is associated with poor cognition: a population-based study of 70-year-old adults without dementia. J Gerontol Ser A 76:2275–2283. https://doi.org/10.1093/gerona/glab195
Davarpanah M, Shokri-mashhadi N, Ziaei R, Saneei P (2021) A systematic review and meta-analysis of association between brain-derived neurotrophic factor and type 2 diabetes and glycemic profile. Sci Rep 11:13773. https://doi.org/10.1038/s41598-021-93271-z
Mainardi M, Fusco S, Grassi C (2015) Modulation of hippocampal neural plasticity by glucose-related signaling. Neural Plast 2015:1–10. https://doi.org/10.1155/2015/657928
Holland WL, Summers SA (2008) Sphingolipids, Insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29:381–402. https://doi.org/10.1210/er.2007-0025
Awad AS, Rouse MD, Khutsishvili K et al (2011) Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 79:1090–1098. https://doi.org/10.1038/ki.2010.544
Kawanabe T, Kawakami T, Yatomi Y et al (2007) Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci 48:53–60. https://doi.org/10.1016/j.jdermsci.2007.06.002
Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J (2021) Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs 81:985–1002. https://doi.org/10.1007/s40265-021-01528-8
McGinley MP, Cohen JA (2021) Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 398:1184–1194. https://doi.org/10.1016/S0140-6736(21)00244-0
Wang X, Yang G (2021) Bone marrow mesenchymal stem cells-derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int 45:775–784. https://doi.org/10.1002/cbin.11522
Miguez A, García-Díaz Barriga G, Brito V et al (2015) Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75 NTR up-regulation and astrocyte-mediated inflammation. Hum Mol Genet 24:4958–4970. https://doi.org/10.1093/hmg/ddv218
Fagan SG, Bechet S, Dev KK (2022) Fingolimod Rescues memory and improves pathological hallmarks in the 3xTg-AD model of Alzheimer’s disease. Mol Neurobiol 59:1882–1895. https://doi.org/10.1007/s12035-021-02613-5
Guitton J, Bandet CL, Mariko ML et al (2020) Sphingosine-1-Phosphate metabolism in the regulation of obesity/type 2 diabetes. Cells 9:1682. https://doi.org/10.3390/cells9071682
Czubowicz K, Jęśko H, Wencel P et al (2019) The role of ceramide and sphingosine-1-phosphate in Alzheimer’s disease and other neurodegenerative disorders. Mol Neurobiol 56:5436–5455. https://doi.org/10.1007/s12035-018-1448-3
Li C, Li J, Kays J et al (2015) Sphingosine 1-phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1-phosphate receptors 1 and/or 3. J Neuroinflammation 12:70. https://doi.org/10.1186/s12974-015-0286-8
Gaire BP, Bae YJ, Choi JW (2019) S1P 1 Regulates M1/M2 polarization toward brain injury after transient focal cerebral ischemia. Biomol Ther (Seoul) 27:522–529. https://doi.org/10.4062/biomolther.2019.005
Bilbo S, Stevens B (2017) Microglia: the brain’s first responders. Cerebrum 2017. http://www.ncbi.nlm.nih.gov/pubmed/30210663. Accessed 15 Oct 2022
Sood A, Preeti K, Fernandes V et al (2021) Glia: a major player in glutamate–GABA dysregulation-mediated neurodegeneration. J Neurosci Res 99:3148–3189. https://doi.org/10.1002/jnr.24977
Bhat SA, Sood A, Shukla R, Hanif K (2019) AT2R Activation prevents microglia pro-inflammatory activation in a NOX-dependent manner: inhibition of PKC activation and p47phox phosphorylation by PP2A. Mol Neurobiol 56:3005–3023. https://doi.org/10.1007/s12035-018-1272-9
Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172. https://doi.org/10.1016/j.bbi.2017.03.003
Liu Z, Ran Y, Qie S et al (2019) Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther 25:1353–1362. https://doi.org/10.1111/cns.13261
Przanowski P, Dabrowski M, Ellert-Miklaszewska A et al (2014) The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl) 92:239–254. https://doi.org/10.1007/s00109-013-1090-5
Liang X, Luo M, Shao B et al (2022) Phosphatidylserine released from apoptotic cells in tumor induces M2-like macrophage polarization through the PSR-STAT3-JMJD3 axis. Cancer Commun 42:205–222. https://doi.org/10.1002/cac2.12272
Kang S, Kim C-H, Jung H et al (2017) Agmatine ameliorates type 2 diabetes induced-Alzheimer’s disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacol 113:467–479. https://doi.org/10.1016/j.neuropharm.2016.10.029
Tabák AG, Herder C, Rathmann W et al (2012) Prediabetes: a high-risk state for diabetes development. Lancet 379:2279–2290. https://doi.org/10.1016/S0140-6736(12)60283-9
Neis VB, Moretti M, Bettio LEB et al (2016) Agmatine produces antidepressant-like effects by activating AMPA receptors and mTOR signaling. Eur Neuropsychopharmacol 26:959–971. https://doi.org/10.1016/j.euroneuro.2016.03.009
American Diabetes Association (2006) Diagnosis and classification of diabetes mellitus. Diabetes Care 29(Suppl 1):S43–S48. https://doi.org/10.1016/B978-0-12-801238-3.65822-1
Muniyappa R, Lee S, Chen H, Quon MJ (2008) Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Metab 294:E15–E26. https://doi.org/10.1152/ajpendo.00645.2007
Cui Y, Yang M, Wang Y et al (2021) Melatonin prevents diabetes-associated cognitive dysfunction from microglia-mediated neuroinflammation by activating autophagy via TLR4/Akt/mTOR pathway. FASEB J 35:e21485. https://doi.org/10.1096/fj.202002247RR
Feng Y, Chu A, Luo Q et al (2018) The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front Pharmacol 9:748. https://doi.org/10.3389/fphar.2018.00748
Johnson LA, Zuloaga KL, Kugelman TL et al (2016) Amelioration of metabolic syndrome-associated cognitive impairments in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine 3:26–42. https://doi.org/10.1016/j.ebiom.2015.12.008
Denninger JK, Smith BM, Kirby ED (2018) Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp. https://doi.org/10.3791/58593
Lueptow LM (2017) Novel Object recognition test for the investigation of learning and memory in mice. J Vis Exp 2017:e55718. https://doi.org/10.3791/55718
Sarnyai Z, Sibille EL, Pavlides C et al (2000) Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proc Natl Acad Sci 97:14731–14736. https://doi.org/10.1073/pnas.97.26.14731
Ardid-Ruiz A, Ibars M, Mena P et al (2019) Resveratrol treatment enhances the cellular response to leptin by increasing OBRb content in palmitate-induced steatotic HepG2 cells. Int J Mol Sci 20:6282. https://doi.org/10.3390/ijms20246282
Bhat SA, Goel R, Shukla S et al (2017) Angiotensin receptor blockade by inhibiting glial activation promotes hippocampal neurogenesis via activation of Wnt/β-catenin signaling in hypertension. Mol Neurobiol 556(55):5282–5298. https://doi.org/10.1007/S12035-017-0754-5
Tian Z, Ren N, Wang J et al (2018) Ginsenoside ameliorates cognitive dysfunction in type 2 diabetic Goto-Kakizaki rats. Med Sci Monit 24:3922–3928. https://doi.org/10.12659/MSM.907417
Li X, Cai Y, Luo J et al (2021) Metformin attenuates hypothalamic inflammation via downregulation of RIPK1-independent microglial necroptosis in diet-induced obese mice. Cell Death Discov 7:338. https://doi.org/10.1038/s41420-021-00732-5
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665. https://doi.org/10.1111/bph.13139
Qin C, Fan W-H, Liu Q et al (2017) Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48:3336–3346. https://doi.org/10.1161/STROKEAHA.117.018505
Tang Y, Li T, Li J et al (2013) Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson’s disease. Cell Death Differ 2014 (part of journal title) 213(21):369–380. https://doi.org/10.1038/cdd.2013.159
He X, Pei S, Meng X et al (2022) Punicalagin attenuates neuronal apoptosis by upregulating 5-hydroxymethylcytosine in the diabetic mouse brain. J Agric Food Chem 70:4995–5004. https://doi.org/10.1021/acs.jafc.2c00863
Zhou W, Yao Y, Li J et al (2019) TIGAR attenuates high glucose-induced neuronal apoptosis via an autophagy pathway. Front Mol Neurosci 12:193. https://doi.org/10.3389/fnmol.2019.00193
Hazari MAH, Ram Reddy B, Uzma N, Santhosh Kumar B (2015) Cognitive impairment in type 2 diabetes mellitus. Int J Diabetes Mellit 3:19–24. https://doi.org/10.1016/j.ijdm.2011.01.001
Rucker JL, McDowd JM, Kluding PM (2012) Executive Function and type 2 diabetes: putting the pieces together. Phys Ther 92:454–462. https://doi.org/10.2522/ptj.20100397
Yermakov LM, Griggs RB, Drouet DE et al (2019) Impairment of cognitive flexibility in type 2 diabetic db/db mice. Behav Brain Res 371:111978. https://doi.org/10.1016/j.bbr.2019.111978
Mastrocola R, Restivo F, Vercellinatto I et al (2005) Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol 187:37–44. https://doi.org/10.1677/joe.1.06269
Benedict C, Grillo CA (2018) Insulin Resistance as a therapeutic target in the treatment of Alzheimer’s disease: a state-of-the-art review. Front Neurosci 12:215. https://doi.org/10.3389/fnins.2018.00215
Takechi R, Lam V, Brook E et al (2017) Blood-Brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: An implication for causal link. Front Aging Neurosci 9:399. https://doi.org/10.3389/fnagi.2017.00399
Carreras I, Aytan N, Choi J-K et al (2019) Dual dose-dependent effects of fingolimod in a mouse model of Alzheimer’s disease. Sci Rep 9:10972. https://doi.org/10.1038/s41598-019-47287-1
Montero ML, Liu J, Orozco J et al (2020) Docosahexaenoic acid protection against palmitic acid-induced lipotoxicity in NGF-differentiated PC12 cells involves enhancement of autophagy and inhibition of apoptosis and necroptosis. J Neurochem 155:559–576. https://doi.org/10.1111/jnc.15038
Ardid-Ruiz A, Ibars M, Mena P et al (2019) Resveratrol treatment enhances the cellular response to leptin by increasing OBRb content in palmitate-induced steatotic HepG2 Cells. Int J Mol Sci 20:49–60. https://doi.org/10.3390/ijms20246282
Garris CS, Wu L, Acharya S et al (2013) Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol 14:1166–1172. https://doi.org/10.1038/ni.2730
Jha MK, Lee W-H, Suk K (2016) Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem Pharmacol 103:1–16. https://doi.org/10.1016/j.bcp.2015.11.003
Espeland MA, Carmichael O, Yasar S et al (2018) Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimer’s Dement 14:1184–1192. https://doi.org/10.1016/j.jalz.2018.05.015
Acknowledgements
All authors are thankful to the Department of Pharmacology and Toxicology, NIPER Hyderabad and Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India.
Funding
This work is supported by National Institute of Pharmaceutical Education and Research, Hyderabad and Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
All authors take responsibility for the integrity and accuracy of this manuscript. Anika Sood, Dharmendra Kumar Khatri, and Shashi Bala Singh contributed to the conceptualization designing and execution of the research; Anika Sood performed studies, evaluated data, and wrote manuscript; Valencia Fernandes, and Kumari Preeti performed studies, evaluated data and reviewed the manuscript; Dharmendra Kumar Khatri, and Shashi Bala Singh reviewed, editied the manuscript, and supervised the research work.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the regulations of the Institutional animal ethics committee (IAEC) NIPER-Hyderabad (Protocol number: NIP/10/2020/PC/384).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sood, A., Fernandes, V., Preeti, K. et al. Fingolimod Alleviates Cognitive Deficit in Type 2 Diabetes by Promoting Microglial M2 Polarization via the pSTAT3-jmjd3 Axis. Mol Neurobiol 60, 901–922 (2023). https://doi.org/10.1007/s12035-022-03120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03120-x