Abstract
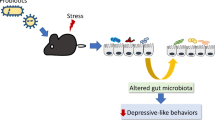
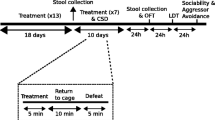
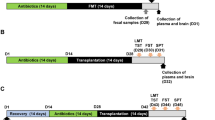
Chronic stress causes maladaptive changes in the brain that lead to depressive behavior. In the present study, we investigate whether chronic stress alters gut microbiota compositions that are related to stress-induced maladaptive changes in the brain. Mice treated with daily 2-h restraint for 14 days (CRST) exhibit depressive-like behavior. Sequence readings of 16S rRNA genes prepared from fecal samples taken from CRST-treated mice suggest that chronic stress induces gut microbiota changes that are pronounced in the post-stress period, relative to those that occur in the 14-day stress phase. The genus Lactobacillus is one such microbiota substantially changed following chronic stress. In contrast, intraperitoneal injection of extracellular vesicles (EVs) isolated from culture media of the Gram-positive probiotic Lactobacillus plantarum is sufficient to ameliorate stress-induced depressive-like behavior. Interestingly, EVs from the Gram-positive probiotic Bacillus subtilis and EVs from the Gram-negative probiotic Akkermansia muciniphila also produce anti-depressive-like effects. While chronic stress decreases the expression of MeCP2, Sirt1, and/or neurotrophic factors in the hippocampus, EVs from the three selected probiotics differentially restore stress-induced changes of these factors. These results suggest that chronic stress produces persistent changes in gut microbiota composition, whereas purified EVs of certain probiotics can be used for treatment of stress-induced depressive-like behavior.






Similar content being viewed by others
Data Availability
Data and materials will be made available on reasonable request.
Code Availability
Not applicable.
Abbreviations
- Bdnf:
-
Brain-derived neurotrophic factor
- CREB:
-
cAMP response element binding protein
- CRST:
-
Chronic restraint stress
- EVs:
-
Extracellular vesicles
- GC:
-
Glucocorticoid
- HDAC2:
-
Histone Deacetylase 2
- IMI:
-
Imipramine
- MeCP2:
-
Methyl CpG binding protein 2
- Ngf:
-
Nerve growth factor
- NT3:
-
Neurotrophin 3
- NT4/5:
-
Neurotrophin 4/5
- OTUs :
-
Operational taxonomic units
- rRNA :
-
Ribosomal RNA
- Sirt1:
-
Sirtuin 1
- TrkB:
-
Tropomyosin receptor kinase B
References
Radley J, Morilak D, Viau V, Campeau S (2015) Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev 58:79–91
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nature Rev Neurosci 6:463–475
McEwen B, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23
Lee EH, Han PL (2019) Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: a systematic review. Neurosci Biobehav Rev 101:13–31
Foster JA, Rinaman L, Cryan JF (2017) Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7;124e136.
Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW (2018) Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol 9:2013
Rea K, Dinan T, Cryan JF (2020) Gut microbiota: a perspective for psychiatrists. Neuropsychobiology 79:50–62
Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS (2020) Altered composition of gut microbiota in depression: a systematic review. Front Psychiatry 11:541
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194
Wallace CJK, Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 16:14
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A (2017) Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 7:43859
Siopi E, Chevalier G, Katsimpardi L, Saha S, Bigot M, Moigneu C, Eberl G, Lledo PM (2020) Changes in gut microbiota by chronic stress impair the efficacy of fluoxetine. Cell Rep 30:3682–3690
Gu F, We Y, Liu Y, Dou M, Jiang Y, Liang H (2020) Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct 11(7):6148–6157
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG (2019) The microbiota-gut-brain axis. Physiol Rev 99(4):1877–2013
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10(11):735–742
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun 8(1):626
Gill S, Catchpole R, Forterre P (2019) Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev 43(3):273–303
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 50(2):e450
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 8(10):e76520
Choi J, Kim YK, Han PL (2019) Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol 28(2):158–171
Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, Lee EH, Kim SW, Lee JK, Kang HS, Han PL (2015) Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis 79:59–69
Choi J, Kim JE, Kim TK, Park JY, Lee JE, Kim H, Lee EH, Han PL (2015) TRH and TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology 97:346–356
Yoo JY, Rho M, You YA, Kwon EJ, Kim MH, Kym S, Jee YK, Kim YK, Kim YJ (2016) 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp Mol Med 48:e208
Kim MH, Rho M, Choi JP, Choi HI, Park HK, Song WJ, Min TK, Cho SH, Cho YJ, Kim YK, Yang S, Pyun BY (2017) A metagenomic analysis provides a culture-independent pathogen detection for atopic dermatitis. Allergy Asthma Immunol Res 9(5):453–461
Kim MH, Choi SJ, Choi HI, Choi JP, Park HK, Kim EK, Kim MJ, Moon BS, Min TK, Rho M, Cho YJ, Yang S, Kim YK, Kim YY, Pyun BY (2018) Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy Asthma Immunol Res 10:516–532
Choi JH, Moon CM, Shin TS, Kim EK, McDowell A, Jo MK, Joo YH, Kim SE, Jung HK, Shim KN, Jung SA, Kim YK (2020) Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med 52:423–437
Kim Y, Edwards N, Fenselau C (2016) Extracellular vesicle proteomes reflect developmental phases of Bacillus subtilis. Clin Proteomics 9(13):6
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 8(10):e76520
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50(2):e450
Choi J, Kwon HJ, Lee JE, Lee Y, Seoh JY, Han PL (2019) Hyperoxygenation revitalizes Alzheimer’s disease pathology through the upregulation of neurotrophic factors. Aging Cell 18(2):e12888
Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL (2012) NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci 32(28):9690–9699
Kim TK, Lee JE, Kim JE, Park JY, Choi J, Kim H, Lee EH, Han PL (2016) G9a-mediated regulation of OXT and AVP expression in the basolateral amygdala mediates stress-induced lasting behavioral depression and its reversal by exercise. Mol Neurobiol 53(5):2843–2856
Lee JE, Kwon HJ, Choi J, Seo JS, Han PL (2020) Aging increases vulnerability to stress-induced depression via upregulation of NADPH oxidase in mice. Commun Biol 3(1):292
Karpova (2014) Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 76 Pt C:709–18.
Al Shoyaib A, Archie SR, Karamyan VT (2019) Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res 37(1):12
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Salvetti E, Torriani S, Felis GE (2012) The genus Lactobacillus: a taxonomic update. Probiotics Antimicrob Proteins 4(4):217–226
Dijl JM, Hecker M (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact 12:3
Payahoo L, Khajebishak Y, Ostadrahimi A (2019) Akkermansia muciniphila bacteria: a new perspective on the management of obesity an updated review. Reviews in Medical Microbiol 30(2):83–89
Davis I, Liu A (2015) What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert Rev Neurother 15(7):719–721
Zhang JC, Yao W (2016) Hashimoto K (2016) Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 14(7):721–731
Funding
This research was supported by a grant (2021R1A2B5B02002245) from the Ministry of Science, ICT and Future Planning, Republic of Korea.
Author information
Authors and Affiliations
Contributions
JC and HK carried out the experiments; YKK provided EVs; JC, HK, and PLH designed the experiments, performed the statistical analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Consent for Publication
All authors consent to the publication of the manuscript in Mol Neurobiol, should the article be accepted by the Editor-in-chief.
Ethics Approval and Consent to Participate
All animals were handled in accordance with the animal care guidelines of Ewha Womans University (IACUC 15–012).
Conflict of Interest
JC, HK, and PLH have no competing financial interests; YKK belongs to MD Healthcare Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, J., Kwon, H., Kim, YK. et al. Extracellular Vesicles from Gram-positive and Gram-negative Probiotics Remediate Stress-Induced Depressive Behavior in Mice. Mol Neurobiol 59, 2715–2728 (2022). https://doi.org/10.1007/s12035-021-02655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02655-9