Abstract
Microglia have been implicated as a key mediator of chronic inflammation following traumatic brain injury (TBI). The animal models of TBI vary significantly based on the type of brain injury (focal versus diffuse). This has made it extremely difficult to assess the role of microglia and the window of microglia activation. Hence, the focus of this review is to summarize the time course of microglia activation in various animal models of TBI. The review explores the repertoire of secondary injury mechanisms such as aberrant neurotransmitter release, oxidative stress, blood-brain barrier disruption, and production of pro-inflammatory cytokines that follow microglia activation. Since receptors act as sensors for activation, we highlight certain microglia receptors that have been implicated in TBI pathology, including fractalkine receptor (CX3CR1), purinergic receptor (P2Y12R), Toll-like receptor (TLR4), scavenger receptors, tumor necrosis factor receptor (TNF-1R), interleukin receptor (IL-1R), complement receptors, and peroxisome proliferator-activated receptor (PPAR). In addition to describing their downstream signaling pathways in TBI, we describe the functional consequences of their activation and the implication in behavioral outcomes. Taken together, this review will provide a holistic view of the role of microglia and its receptors in TBI based on animal studies.


Similar content being viewed by others
Abbreviations
- Akt:
-
protein kinase B
- AP-1:
-
activator protein-1
- APP:
-
amyloid precursor protein
- Arg-1:
-
arginase-1
- ATP:
-
adenosine triphosphate
- BBB:
-
blood-brain barrier
- CCI:
-
controlled cortical impact/injury
- cFPI:
-
central FPI
- cIAP-1:
-
cellular inhibitor of apoptosis protein-1
- CNS:
-
central nervous system
- CR:
-
complement receptor
- CX3CR1:
-
chemokine receptor 1
- CXCL1:
-
fractalkine
- DAMPS:
-
danger associated molecular patterns
- EC:
-
endothelial cells
- FPI:
-
fluid percussion injury
- GOAD:
-
Gila open access database
- HAPI:
-
highly aggressively proliferating immortalized
- HMGB1:
-
high mobility group box protein 1
- HSP:
-
heat shock protein
- IL-1R1:
-
interleukin 1 receptor
- IL-1β:
-
interleukin 1β
- IRAK4:
-
IL-1R associated kinase 4
- IRF-3:
-
interferon regulatory factor–3
- KO:
-
knockout
- LFPI:
-
lateral FPI
- LPA:
-
lysophosphatidic acid
- LPS:
-
lipopolysaccharide
- MAPK:
-
mitogen-activated protein kinase
- MIP-1α:
-
macrophage inflammatory protein-1α
- mRNA:
-
messenger RNA
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- NFκB:
-
nuclear factor-kappa B
- NOX:
-
NADPH oxidase
- PACAP30:
-
adenylate cyclase-activating polypeptide
- PAMPS:
-
pathogens associated molecular patterns
- PCR:
-
polymerase chain reaction
- PPAR:
-
peroxisome proliferator-activated receptors
- RAGE:
-
receptor for advanced glycation end products
- RIP:
-
receptor-interacting protein
- RNA:
-
ribonucleic acid
- RNA-seq:
-
RNA sequencing
- RNS:
-
reactive nitrogen species
- ROS:
-
reactive oxygen species
- STAT:
-
signal transducer and activator of transcription
- TBI:
-
traumatic brain injury
- TLR4:
-
Toll-like receptor 4
- TNF α:
-
tumor necrosis factor α
- TNF-R1:
-
TNFα receptor 1
- TRADD:
-
TNF receptor-associated death domain
- TRAF-2:
-
TNF receptor-associated factor-2
- TSPO:
-
translocator protein
- WD:
-
weight drop
References
Taylor CA et al (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ 66(9):1–18
Cernak I, Noble-Haeusslein LJ (2010) Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab 30(2):255–266
Faul MX, Likang, Wald M, Coronado V (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006, C.f.D.C.a. Prevention, Editor. National Center for Injury Prevention and Control, Atlanta
Maas AIR, Roozenbeek B, Manley GT (2010) Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics 7(1):115–126
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Huber BR et al (2016) Blast exposure causes dynamic microglial/macrophage responses and microdomains if brain microvessel dysfunction. Neuroscience 319:206–220
Loane DJ, Kumar A (2016) Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol 275:316–327
Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39(1):3–18
Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal-macrophages—comparison of activating cytokines and evidence for independent production. J Immunol 141(7):2407–2412
Sica A et al (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42(6):717–727
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991
Ginhoux F et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845
Schulz C et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336(6077):86–90
Link VM, Gosselin D, Glass CK (2015) Mechanisms underlying the selection and function of macrophage-specific enhancers. Cold Spring Harb Symp Quant Biol 80:213–221
Butovsky O et al (2014) Identification of a unique TGF-beta dependent molecular and functional signature in microglia. Nat Neurosci 17(1):131–143
Cherry JD, Olschowka JA, O'Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:15
Chiu CC et al (2016) Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods 272:38–49
Deselms H et al (2016) Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development. J Neurosci Methods 272:69–76
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142
Dixon CE et al (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39(3):253–262
Lighthall JW (1988) Controlled cortical impact: a new experimental brain injury model. J Neurotrauma 5(1):1–15
Smith DH et al (1995) A model of parasagittal controlled cortical impact in the mouse—cognitive and histopathologic effects. J Neurotrauma 12(2):169–178
Lighthall JW, Goshgarian HG, Pinderski CR (1990) Characterization of axonal injury produced by controlled cortical impact. J Neurotrauma 7(2):65–76
Garman RH et al (2011) Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma 28(6):947–959
Jin XM et al (2012) Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One 7(7):13
Loane DJ et al (2014) Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol 73(1):14–29
Bachstetter AD et al (2013) The p38 alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci 33(14):6143–6153
Cao T et al (2012) Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 225:65–75
Zhang ZR et al (2012) Immunolocalization of Toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation 19(1):10–19
Zhang ZY et al (2007) Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol 113(6):675–682
Kaur C et al (1995) The response of neurons and microglia to blast injury in the rat-brain. Neuropathol Appl Neurobiol 21(5):369–377
Morganti JM, Riparip LK, Rosi S (2016) Call off the dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS One 11(1):13
Urrea C et al (2007) Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci 25(1):65–76
Ziebell JM et al (2012) Rod microglia: Elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation 9:11
Huang EYK et al (2014) Remote effects on the striatal dopamine system after fluid percussion injury. Behav Brain Res 267:156–172
Carbonnel WS, Grady MS (1999) Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol 98(4):396–406
Morrison H et al (2017) Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep 7:12
Kelley BJ, Lifshitz J, Povlishock JT (2007) Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol 66(11):989–1001
Readnower RD et al (2010) Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res 88(16):3530–3539
Xu LY et al (2016) Neuroinflammation in primary blast neurotrauma: time course and prevention by torso shielding. Exp Neurol 277:268–274
Israel I et al (2016) Combined F-18 DPA-714 micro-positron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. J Neuroinflammation 13:13
Kabadi SV et al (2015) S100B inhibition reduces behavioral and pathologic changes in experimental traumatic brain injury. J Cereb Blood Flow Metab 35(12):2010–2020
Kumar A et al (2016) Microglial/macrophage polarization dynamics following traumatic brain injury. J Neurotrauma 33(19):1732
Bachstetter AD et al (2011) Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ). J Neuroinflammation 8(1):79
Mastroeni D et al (2009) Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol Aging 30(11):1805–1817
Kumar A et al (2013) Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34(5):1397–1411
Yao XL et al (2017) TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol 310:38–45
Rao VLR et al (2000) Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol 161(1):102–114
Yi JH et al (2008) PPAR gamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 1244:164–172
Sipe GO et al (2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun 7:15
Balcaitis S et al (2003) Expression of proteinase-activated receptors in mouse microglial cells. Neuroreport 14(18):2373–2377
Mitrasinovic OM, Murphy GM (2002) Accelerated phagocytosis of amyloid-beta by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor. J Biol Chem 277(33):29889–29896
Mitrasinovic OM, Murphy GM (2003) Microglial overexpression of the M-CSF receptor augments phagocytosis of opsonized A beta. Neurobiol Aging 24(6):807–815
Mitrasinovic OM et al (2003) Macrophage colony stimulating factor promotes phagocytosis by murine microglia. Neurosci Lett 344(3):185–188
Wang XJ et al (2007) CD200-CD200R regulation of microglia activation in the pathogenesis of Parkinson’s disease. J NeuroImmune Pharmacol 2(3):259–264
Moller T et al (2001) Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J Biol Chem 276(28):25946–25952
Tiffany HL et al (2001) Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J Biol Chem 276(26):23645–23652
Cui YH et al (2002) Bacterial lipopolysaccharide selectively up-regulates the function of the chemotactic peptide receptor formyl peptide receptor 2 in murine microglial cells. J Immunol 168(1):434–442
Hall AA et al (2009) Sigma receptors suppress multiple aspects of microglial activation. Glia 57(7):744–754
Gekker G et al (2006) Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transfonning growth factor-beta 1. Int Immunopharmacol 6(6):1029–1033
Truettner JS, Bramlett HM, Dietrich WD (2017) Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab 37(8):2952–2962
Wen L et al (2018) Polarization of microglia to the M2 phenotype in a PPAR-gamma dependent manner attenuates axonal injury induced by traumatic brain injury in mice. J Neurotrauma
Wang GH et al (2013) Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 33(12):1864–1874
Bollmann L et al (2015) Microglia mechanics: Immune activation alters traction forces and durotaxis. Front Cell Neurosci 9:16
Navone SE et al (2018) Mechanical loading of intervertebral disc modulates microglia proliferation, activation, and chemotaxis. Osteoarthr Cartil 26(7):978–987
Mittelbronn M et al (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 101(3):249–255
Igarashi T, Huang TT, Noble LJ (2001) Regional vulnerability after traumatic brain injury: Gender differences in mice that overexpress human copper, zinc superoxide dismutase. Exp Neurol 172(2):332–341
Tong W et al (2002) Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol 176(1):105–116
Gyoneva S et al (2015) Ccr2 deletion dissociates cavity size and tau pathology after mild traumatic brain injury. J Neuroinflammation 12:12
Kelso ML, Gendelman HE (2014) Bridge between neuroimmunity and traumatic brain injury. Curr Pharm Des 20(26):4284–4298
Abe N et al (2018) Comparison of the detrimental features of microglia and infiltrated macrophages in traumatic brain injury: a study using a hypnotic bromovalerylurea. Glia
Sedgwick JD et al (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88(16):7438–7442
Ransohoff RM (2011) Microglia and monocytes: 'tis plain the twain meet in the brain. Nat Neurosci 14(9):1098–1100
Hsieh CL et al (2013) Traumatic brain injury induces macrophage subsets in the brain. Eur J Immunol 43(8):2010–2022
Morganti JM et al (2015) CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci 35(2):748–760
Hsieh CL et al (2014) CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J Neurotrauma 31(20):1677–1688
Prins M et al (2013) The pathophysiology of traumatic brain injury at a glance. Dis Model Mech 6(6):1307–1315
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99(1):4–9
Barger SW, Harmon AD (1997) Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 388(6645):878–881
Noda M, Nakanishi H, Akaike N (1999) Glutamate release from microglia via glutamate transporter is enhanced by amyloid-beta peptide. Neuroscience 92(4):1465–1474
Barger SW, Basile AS (2001) Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem 76(3):846–854
Faden AI et al (1989) The role of excitatory amino-acids and Nmda receptors in traumatic brain injury. Science 244(4906):798–800
Christensen RN et al (2006) Kainate induces rapid redistribution of the actin cytoskeleton in ameboid microglia. J Neurosci Res 84(1):170–181
Hagino Y et al (2004) Heterogeneity and potentiation of AMPA type of glutamate receptors in rat cultured microglia. Glia 47(1):68–77
Noda M et al (2000) AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 20(1):251–258
Biber K et al (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 72(4):1671–1680
Taylor DL et al (2002) Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer’s disease. J Neurochem 82(5):1179–1191
Taylor DL, Diemel LT, Pocock JM (2003) Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci 23(6):2150–2160
Taylor DL et al (2005) Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived fas ligand. J Neurosci 25(11):2952–2964
Murugan M et al (2011) Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-kappa B signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats. Glia 59(4):521–539
Liu GJ et al (2006) Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol Pharmacol 70(3):851–859
Gottlieb M, Matute C (1997) Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab 17(3):290–300
Ferrari D et al (1997) ATP-mediated cytotoxicity in microglial cells. Neuropharmacology 36(9):1295–1301
Ferrari D et al (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med 185(3):579–582
Fujita R, Ma Y, Ueda H (2008) Lysophosphatidic acid-induced membrane ruffling and brain-derived neurotrophic factor gene expression are mediated by ATP release in primary microglia. J Neurochem 107(1):152–160
Ballerini P et al (2005) P2Y(1) and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int J Immunopathol Pharmacol 18(2):255–268
Xu PF et al (2015) Extracellular ATP enhances radiation-induced brain injury through microglial activation and paracrine signaling via P2X7 receptor. Brain Behav Immunity 50:87–100
Lou NH et al (2016) Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A 113(4):1074–1079
Koizumi S et al (2007) UDP acting at P2Y(6) receptors is a mediator of microglial phagocytosis. Nature 446(7139):1091–1095
Koizumi S et al (2013) Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 61(1):47–54
Hidetoshi TS, Makoto T, Inoue K (2012) P2Y receptors in microglia and neuroinflammation. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1(4):493–501
del Puerto A, Wandosell F, Garrido JJ (2013) Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front Cell Neurosci 7:15
Ozdemir D et al (2005) Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res 54(6):631–637
Solaroglu I et al (2005) Increased xanthine oxidase activity after traumatic brain injury in rats. J Clin Neurosci 12(3):273–275
Lewen A, Matz P, Chan PH (2000) Free radical pathways in CNS injury. J Neurotrauma 17(10):871–890
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 87(1):245–313
Sankarapandi S et al (1998) Measurement and characterization of superoxide generation in microglial cells: Evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys 353(2):312–321
Nauseef WM (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102
Kumar A et al (2016) NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain behavior and Immunity 58:291–309
Ferreira APO et al (2013) The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochem Int 63(6):583–593
Rao KVR et al A single primary blast-induced traumatic brain injury in a rodent model causes cell-type dependent increase in nicotinamide adenine dinucleotide phosphate oxidase isoforms in vulnerable brain regions. J Neurotrauma:14
Petitto JN et al (2003) IL-2 gene knockout affects T lymphocyte trafficking and the microglial response to regenerating facial motor neurons. J Neuroimmunol 134(1–2):95–103
Sakai N, Kaufman S, Milstien S (1995) Parallel induction of nitric-oxide and tetrahydrobiopterin synthesis by cytokines in rat glial-cells. J Neurochem 65(2):895–902
Hanisch UK et al (1997) Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to janus kinase activity. J Biol Chem 272(46):28853–28860
Bruce AJ et al (1996) Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 2(7):788–794
Moller T, Hanisch UK, Ransom BR (2000) Thrombin-induced activation of cultured rodent microglia. J Neurochem 75(4):1539–1547
Cao Q et al (2008) Expression of Notch-1 receptor and its ligands Jagged-1 and Delta-1 in amoeboid microglia in postnatal rat brain and murine BV-2 cells. Glia 56(11):1224–1237
Grandbarbe L et al (2007) Notch signaling modulates the activation of microglial cells. Glia 55(15):1519–1530
Farber K, Pannasch U, Kettenmann H (2005) Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci 29(1):128–138
Miyoshi M et al (2008) Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappa B and activator protein-1 activation. Eur J Neurosci 27(2):343–351
Zhao X et al (2018) Microglial interactions with the neurovascular system in physiology and pathology. Dev Neurobioy
Stankovic ND et al (2016) Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol 131(3):347–363
Thal SC, Neuhaus W (2014) The blood-brain barrier as a target in traumatic brain injury treatment. Arch Med Res 45(8):698–710
Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011) Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2(4):492–516
Shlosberg D et al (2010) Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6(7):393–403
Tchantchou F, Zhang YM (2013) Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma 30(7):565–579
Choi BY et al (2012) Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res 1481:49–58
Kuriakose M et al (2018) Synergistic roles of oxidative stress and blood-brain barrier permeability as injury mechanisms in the acute pathophysiology of blast-induced neurotrauma. J Neurotrauma under review
Trahanas DM et al (2015) Differential activation of infiltrating monocyte-derived cells after mild and severe traumatic brain injury. Shock 43(3):255–260
Shechter R et al (2009) Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6(7):17
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155
Febinger HY et al (2015) Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J Neuroinflammation 12:16
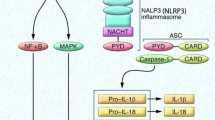
Qian HH, Li QH, Shi WD (2017) Hyperbaric oxygen alleviates the activation of NLRP-3-inflammasomes in traumatic brain injury. Mol Med Rep 16(4):3922–3928
Chio CC et al (2013) Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci 14:12
Pinteaux-Jones F et al (2008) Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J Neurochem 106(1):442–454
McMullan SM et al (2012) Metabotropic glutamate receptors inhibit microglial glutamate release. Asn Neuro 4(5):323–330
Mori K et al (2002) Effects of norepinephrine on rat cultured microglial cells that express alpha 1, alpha 2, beta 1 and beta 2 adrenergic receptors. Neuropharmacology 43(6):1026–1034
Farez MF et al (2009) Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol 10(9):958–U44
Jack CS et al (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175(7):4320–4330
Suuronen T et al (2006) Characterization of the pro-inflammatory signaling induced by protein acetylation in microglia. Neurochem Int 49(6):610–618
Kielian T, Mayes P, Kielian M (2002) Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol 130(1–2):86–99
Charles KJ et al (2003) GABA(B) receptor subunit expression in glia. Mol Cell Neurosci 24(1):214–223
Harry GJ et al (2008) Tumor necrosis factor p55 and p75 receptors are involved in chemical-induced apoptosis of dentate granule neurons. J Neurochem 106(1):281–298
Kettenmann H et al (2011) Physiology of microglia. Physiol Rev 91(2):461–553
Hausler KG et al (2002) Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur J Neurosci 16(11):2113–2122
Lee YB, Nagai A, Kim SU (2002) Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res 69(1):94–103
van Rossum D et al (2008) Myelin-phagocytosing macrophages in isolated sciatic and optic nerves reveal a unique reactive phenotype. Glia 56(3):271–283
McIntosh TK et al (1996) Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab Investig 74(2):315–342
Kumar A, Loane DJ (2012) Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immunity 26(8):1191–1201
Loane DJ, Faden AI (2010) Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci 31(12):596–604
Basrai HS et al (2016) Suppressor of cytokine signaling-2 (SOCS2) regulates the microglial response and improves functional outcome after traumatic brain injury in mice. PLoS One 11(4):24
Clausen F et al (2009) Neutralization of interleukin-1 beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 30(3):385–396
Dong H et al (2016) Sigma-1 receptor modulates neuroinflammation after traumatic brain injury. Cell Mol Neurobiol 36(5):639–645
Elliott MB et al (2011) Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J Neurotrauma 28(6):973–981
Erturk A et al (2016) Interfering with the chronic immune response rescues chronic degeneration after traumatic brain injury. J Neurosci 36(38):9962–9975
Zanier ER et al (2016) Fractalkine receptor deficiency is associated with early protection but late worsening of outcome following brain trauma in mice. J Neurotrauma 33(11):1060–1072
Haselkorn ML et al (2010) Adenosine a(1) receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J Neurotrauma 27(5):901–910
Laird MD et al (2014) High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 62(1):26–38
Loane D et al (2014) Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics 11(4):857–869
Loane DJ et al (2013) Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J Neurotrauma 30(5):403–412
Longhi L et al (2013) Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor. J Cereb Blood Flow Metab 33(8):1182–1189
Venneti S et al (2007) The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: Implications for PET imaging. Exp Neurol 207(1):118–127
Liu H et al (2016) Rosiglitazone attenuates inflammation and CA3 neuronal loss following traumatic brain injury in rats. Biochem Biophys Res Commun 472(4):648–655
Sauerbeck A et al (2011) Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol 227(1):128–135
Gao WW et al (2015) VEGI attenuates the inflammatory injury and disruption of blood-brain barrier partly by suppressing the TLR4/NF-kappa B signaling pathway in experimental traumatic brain injury. Brain Res 1622:230–239
Ekmark-Lewen S et al (2016) Diffuse traumatic axonal injury in mice induces complex behavioural alterations that are normalized by neutralization of interleukin-1. Eur J Neurosci 43(8):1016–1033
Pilipovic K et al (2015) A single dose of PPAR gamma agonist pioglitazone reduces cortical oxidative damage and microglial reaction following lateral fluid percussion brain injury in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 59:8–20
Bye N et al (2007) Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol 204(1):220–233
Chang CZ et al (2015) Magnesium lithospermate B implicates 3 '-5 '-cyclic adenosine monophosphate/protein kinase a pathway and N-methyl-D-aspartate receptors in an experimental traumatic brain injury. World Neurosurgery 84(4):954–963
Hellewell SC et al (2013) Erythropoietin improves motor and cognitive deficit, axonal pathology, and neuroinflammation in a combined model of diffuse traumatic brain injury and hypoxia, in association with upregulation of the erythropoietin receptor. J Neuroinflammation 10:21
Mao SS et al (2012) Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-kappa B pathway. J Neurotrauma 29(10):1941–1959
Wang JW et al (2013) Activation of metabotropic glutamate receptor 5 reduces the secondary brain injury after traumatic brain injury in rats. Biochem Biophys Res Commun 430(3):1016–1021
Wang JW et al (2012) Expression and cell distribution of metabotropic glutamate receptor 5 in the rat cortex following traumatic brain injury. Brain Res 1464:73–81
Khuman J et al (2011) Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab 31(2):778–789
Bu W et al (2016) Mild traumatic brain injury produces neuron loss that can be rescued by modulating microglial activation using a CB2 receptor inverse agonist. Front Neurosci 10:17
Reiner A et al (2015) Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int J Mol Sci 16(1):758–787
Cardona AE et al (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924
Tarozzo G et al (2002) Expression of fractalkine and its receptor, CX(3)CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci 15(10):1663–1668
Harrison JK et al (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95(18):10896–10901
Clark AK et al (2007) Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A 104(25):10655–10660
Zhuang ZY et al (2007) Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain behavior and Immunity 21(5):642–651
Boehme SA et al (2000) The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol 165(1):397–403
Abbracchio MP, Burnstock G (1994) Purinoceptors—are there families of P2X and P2Y Purinoceptors. Pharmacol Ther 64(3):445–475
Sasaki Y et al (2003) Selective expression of Gi/o-coupled ATP receptor P2Y(12) in microglia in rat brain. Glia 44(3):242–250
Honda S et al (2001) Extracellular ATP or ADP induce chemotaxis of cultured microglia through G(i/o)-coupled P2Y receptors. J Neurosci 21(6):1975–1982
Madry C et al (2018) Microglial ramification, surveillance, and Interleukin-1 beta release are regulated by the two-pore domain K+ channel THIK-1. Neuron 97(2):299
Swiatkowski P et al (2016) Activation of microglial P2Y12 receptor is required for outward potassium currents in response to neuronal injury. Neuroscience 318:22–33
Moore CS et al (2015) P2Y12 expression and function in alternatively activated human microglia. Neurology-Neuroimmunology & Neuroinflammation 2(2):10
Tozaki-Saitoh H et al (2017) P2Y12 receptors in primary microglia activate nuclear factor of activated T-cell signaling to induce C-C chemokine 3 expression. J Neurochem 141(1):100–110
Kobayashi K et al (2008) P2Y(12) receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28(11):2892–2902
Gu N et al (2016) Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immunity 55:82–92
Avignone E et al (2008) Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci 28(37):9133–9144
Haynes SE et al (2006) The P2Y(12) receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9(12):1512–1519
Sieger D et al (2012) Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell 22(6):1138–1148
Lafrenaye AD et al (2015) Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J Neuroinflammation 12:15
Eyo UB et al (2015) Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci 35(6):2417–2422
Kato G et al (2016) Microglial contact prevents excess depolarization and rescues neurons from excitotoxicity. Eneuro 3(3):9
Esen N, Kielian T (2006) Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol 176(11):6802–6811
Zhang Z, Schluesener HJ (2006) Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci 63(24):2901–2907
Ock J et al (2007) Regulation of toll-like receptor 4 expression and its signaling by hypoxia in cultured microglia. J Neurosci Res 85(9):1989–1995
El Khoury J et al (1998) Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging 19(1 Suppl):S81–S84
Wilkinson K, El Khoury J (2012) Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer's disease. Int J Alzheimers Dis 2012:489456
El Khoury JB et al (2003) CD36 mediates the innate host response to β-amyloid. J Exp Med 197(12):1657–1666
Yehualaeshet T et al (1999) Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol 155(3):841–851
Means TK et al (2009) Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med 206(3):637–653
Stewart CR et al (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11(2):155–161
Moore KJ et al (2002) A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem 277(49):47373–47379
Wilkinson B et al (2006) Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem 281(30):20842–20850
Cho S et al (2005) The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci 25(10):2504–2512
Sheedy FJ et al (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat Immunol 14(8):812–820
Woo MS et al (2016) Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem 291(45):23654–23661
Lue LF et al (2001) Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol 171(1):29–45
Gao TL et al (2012) Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J Trauma Acute Care Surg 72(3):643–649
Weber DJ et al (2015) The HMGB1-RAGE inflammatory pathway: implications for brain injury-induced pulmonary dysfunction. Antioxid Redox Signal 23(17):1316–1328
Wang WY et al (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136
Fang F et al (2010) RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J 24(4):1043–1055
Weber DJ et al (2014) The HMGB1-RAGE axis mediates traumatic brain injury–induced pulmonary dysfunction in lung transplantation. Sci Transl Med 6(252):252ra124–252ra124
Wang H et al (2017) HMGB1/advanced glycation end products (RAGE) does not aggravate inflammation but promote endogenous neural stem cells differentiation in spinal cord injury. Sci Rep 7(1):10332
Zhang Y et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947
Sims JE, Smith DE (2010) The IL-1 family: regulators of immunity. Nat Rev Immunol 10(2):89–102
Bruttger J et al (2015) Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43(1):92–106
Stavridis S et al (2005) Characterisation of transverse slice culture preparations of postnatal rat spinal cord: preservation of defined neuronal populations. Histochem Cell Biol 123(4–5):377–392
Basu A et al (2002) The type 1 Interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J Neurosci 22(14):6071
Robson MJ et al (2016) Generation and characterization of mice expressing a conditional allele of the Interleukin-1 receptor type 1. PLoS One 11(3):17
Ma J et al (2016) Propofol inhibits NLRP3 Inflammasome and attenuates blast-induced traumatic brain injury in rats. Inflammation 39(6):2094–2103
Fan KH et al (2017) Mangiferin attenuates blast-induced traumatic brain injury via inhibiting NLRP3 inflammasome. Chem Biol Interact 271:15–23
Halle A et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8):857–865
Hanamsagar R, Torres V, Kielian T (2011) Inflammasome activation and IL-1 beta/IL-18 processing are influenced by distinct pathways in microglia. J Neurochem 119(4):736–748
Shi FS et al (2013) Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptide-induced NALP3 inflammasome activation in BV2 microglia. J Neuroimmunol 260(1–2):121–125
Duncan JA et al (2009) Neisseria gonorrhoeae activates the proteinase Cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing Inflammasome. J Immunol 182(10):6460–6469
Ichinohe T et al (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206(1):79–87
Cruz CM et al (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282(5):2871–2879
Hoegen T et al (2011) The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal Cathepsin B release. J Immunol 187(10):5440–5451
Mariathasan S et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–232
Martinon F et al (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–241
Hornung V et al (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9(8):847–856
Lee SW et al (2018) Microglial inflammasome activation in penetrating ballistic-like brain injury. J Neurotrauma:13
Liu HD et al (2013) Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 38(10):2072–2083
Kuno R et al (2005) Autocrine activation of microglia by tumor necrosis factor-alpha. J Neuroimmunol 162(1–2):89–96
Kraft AD, McPherson CA, Harry GJ (2009) Heterogeneity of microglia and TNF signaling as determinants for neuronal death or survival. Neurotoxicology 30(5):785–793
Chen GQ, Goeddel DV (2002) TNF-R1 signaling: A beautiful pathway. Science 296(5573):1634–1635
Liu C, Tang JW (2014) Expression levels of tumor necrosis factor-alpha and the corresponding receptors are correlated with trauma severity. Oncol Lett 8(6):2747–2751
Yang JS et al (2010) Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice. J Neurotrauma 27(6):1037–1046
Longhi L et al (2008) Effect of traumatic brain injury on cognitive function in mice lacking p55 and p75 tumor necrosis factor receptors. In: Manley G, Hemphill C, Stiver S (eds) Intracranial pressure and brain monitoring Xiii: mechanisms and treatment. Springer-Verlag, Wien, p. 409
Lotocki G et al (2004) Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci 24(49):11010–11016
Yager PH et al (2008) Mannose binding lectin gene deficiency increases susceptibility to traumatic brain injury in mice. J Cereb Blood Flow Metab 28(5):1030–1039
Hailer NP (2008) Immunosuppression after traumatic or ischemic CNS damage: It is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol 84(3):211–233
Bermpohl D et al (2007) TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab 27(11):1806–1818
Gasque P et al (1998) The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol 160(7):3543–3554
Stahel PF et al (2000) Intracerebral complement C5a receptor (CD88) expression is regulated by TNF and lymphotoxin-alpha following closed head injury in mice. J Neuroimmunol 109(2):164–172
Osaka H et al (1999) Expression of C5a receptor in mouse brain: role in signal transduction and neurodegeneration. Neuroscience 88(4):1073–1082
Fumagalli S et al (2015) The ischemic environment drives microglia and macrophage function. Front Neurol 6:19
Hajishengallis G et al (2007) Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol 179(4):2359–2367
Kohl J (2006) The role of complement in danger sensing and transmission. Immunol Res 34(2):157–176
Hammad A, Westacott L, Zaben M (2018) The role of the complement system in traumatic brain injury: a review. J Neuroinflammation 15:24
Veerhuis R, Nielsen HM, Tenner AJ (2011) Complement in the brain. Mol Immunol 48(14):1592–1603
Crehan H, Hardy J, Pocock J (2012) Microglia, Alzheimer’s disease, and complement. Int J Alzheimers Dis 2012:983640
Brennan FH et al (2012) Complement activation in the injured central nervous system: another dual-edged sword? J Neuroinflammation 9:137
Keeling KL et al (2000) Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the complement system. J Neuroimmunol 105(1):20–30
Bellander BM et al (2011) Secondary insults following traumatic brain injury enhance complement activation in the human brain and release of the tissue damage marker S100B. Acta Neurochir 153(1):90–100
Kossmann T et al (1997) Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol 73(1–2):63–69
Kaczorowski SL et al (1995) Effect of soluble complement receptor-1 on neutrophil accumulation after traumatic brain injury in rats. J Cereb Blood Flow Metab 15(5):860–864
Yang S et al (2006) The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab 26(12):1490–1495
Rancan M et al (2003) Central nervous system-targeted complement inhibition mediates neuroprotection after closed head injury in transgenic mice. J Cereb Blood Flow Metab 23(9):1070–1074
Neher MD et al (2014) Deficiency of complement receptors CR2/CR1 in Cr2(−)/(−) mice reduces the extent of secondary brain damage after closed head injury. J Neuroinflammation 11:95
Leinhase I et al (2006) Pharmacological complement inhibition at the C3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Exp Neurol 199(2):454–464
Garrett MC et al (2009) Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res 1298:171–177
Michalik L, Wahli W (1999) Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Curr Opin Biotechnol 10(6):564–570
Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169(3):453–459
Besson VC et al (2005) Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett 388(1):7–12
Chen XR et al (2007) Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma 24(7):1119–1131
Park SW et al (2007) Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320(3):1002–1012
Hyong A et al (2008) Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res 1215:218–224
Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci Landmark 13:1813–1826
Hunter RL et al (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100(5):1375–1386
McTigue DM et al (2007) The PPAR gamma agonist pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol 205(2):396–406
Warden A et al (2016) Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep 6:15
Kust BM et al (1999) Regulation of K+ channel mRNA expression by stimulation of adenosine A(2a)-receptors in cultured rat microglia. Glia 25(2):120–130
Fiebich BL et al (1996) Cyclooxygenase-2 expression in rat microglia is induced by adenosine A(2a)-receptors. Glia 18(2):152–160
Heese K et al (1997) Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A(2a)-receptors. Neurosci Lett 231(2):83–86
Hammarberg C, Schulte G, Fredholm BB (2003) Evidence for functional adenosine A(3) receptors in microglia cells. J Neurochem 86(4):1051–1054
Xiang ZH, Burnstock G (2005) Expression of P2X receptors on rat microglial cells during early development. Glia 52(2):119–126
Horvath RJ, Deleo JA (2009) Morphine enhances microglial migration through modulation of P2X(4) receptor signaling. J Neurosci 29(4):998–1005
Cavaliere F, Dinkel K, Reymann K (2005) Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience 136(3):615–623
Visentin S, Nuccio CD, Bellenchi GC (2006) Different patterns of Ca+ signals are induced by low compared to high concentrations of P2Y agonists in microglia. Purinergic Signalling 2(4):605–617
Light AR et al (2006) Purinergic receptors activating rapid intracellular Ca2+ increases in microglia. Neuron Glia Biol 2:125–138
Liu GJ et al (2009) Glutamate induces directed chemotaxis of microglia. Eur J Neurosci 29(6):1108–1118
Drouin-Ouellet J et al (2011) Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia 59(2):188–199
Geurts JJG et al (2003) Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 126:1755–1766
Byrnes KR et al (2009) Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57(5):550–560
Loane DJ et al (2009) Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284(23):15629–15639
Kuhn SA et al (2004) Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci 25(2):312–322
De Simone R et al (2005) Activation of alpha 7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E-2 in rat microglial cultures. J Neuroinflamm 2:10
Shytle RD et al (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89(2):337–343
Suzuki T et al (2006) Microglial alpha 7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res 83(8):1461–1470
Rock RB et al (2008) Potentiation of HIV-1 expression in microglial cells by nicotine: Involvement of transforming growth factor-beta 1. J NeuroImmune Pharmacol 3(3):143–149
Liu J et al (2009) Nicotinic acetylcholine receptor subunits in rhesus monkey retina. Invest Ophthalmol Vis Sci 50(3):1408–1415
Fujita H et al (1998) Adrenergic agonists suppress the proliferation of microglia through beta 2-adrenergic receptor. Neurosci Lett 242(1):37–40
Mori M et al (1996) Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci 16(11):3590–3600
Sattayaprasert P et al (2005) Platelet-activating factor enhancement of calcium influx and interleukin-6 expression, but not production, in human microglia. J Neuroinflamm 2:8
Wang X et al (1999) Platelet-activating factor induced Ca2+ signaling in human microglia. Brain Res 842(1):159–165
Noda M et al (2004) Kinin receptors in cultured rat microglia. Neurochem Int 45(2–3):437–442
Noda M et al (2007) Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. J Neurochem 101(2):397–410
Ifuku M et al (2007) Bradykinin-induced microglial migration mediated by B-1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci 27(48):13065–13073
Noda M et al (2003) Expression and function of bradykinin receptors in microglia. Life Sci 72(14):1573–1581
Bader MF et al (1994) Bacterial-endotoxin induces Ca2+ I transients and changes the Organization of Actin in microglia. Glia 11(4):336–344
McLarnon JG et al (1999) Endothelin-induced changes in intracellular calcium in human microglia. Neurosci Lett 263(1):9–12
Moller T et al (1997) Endothelin-induced calcium signaling in cultured mouse microglial cells is mediated through ETB receptors. Neuroreport 8(9–10):2127–2131
Yamashita K et al (1994) Microglia with an endothelin et(B) receptor aggregate in rat Hippocampus Ca1 subfields following transient forebrain ischemia. J Neurochem 63(3):1042–1051
Cabral GA, Marciano-Cabral F (2005) Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol 78(6):1192–1197
Facchinetti F et al (2003) Cannabinoids ablate release of TNF alpha in rat microglial cells stimulated with lypopolysaccharide. Glia 41(2):161–168
Ramirez BG et al (2005) Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25(8):1904–1913
Maresz K et al (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95(2):437–445
Feindt J, Schmidt A, Mentlein R (1998) Receptors and effects of the inhibitory neuropeptide somatostatin in microglial cells. Mol Brain Res 60(2):228–233
Tanaka J et al (1997) Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia 20(1):23–37
Chao CC et al (1996) Kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci U S A 93(15):8051–8056
Dobrenis K, Makman MH, Stefano GB (1995) Occurrence of the opiate alkaloid-selective mu(3) receptor in mammalian microglia, astrocytes and Kupffer cells. Brain Res 686(2):239–248
Lai JP et al (2000) Detection of substance P and its receptor in human fetal microglia. Neuroscience 101(4):1137–1144
Rasley A et al (2002) Expression of functional NK-1 receptors in murine microglia. Glia 37(3):258–267
Delgado M, Ganea D (2003) Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J 17(11):1922
Delgado M, Jonakait GM, Ganea D (2002) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia 39(2):148–161
Gonzalez-Rey E, Delgado M (2008) Vasoactive intestinal peptide inhibits cycloxygenase-2 expression in activated macrophages, microglia, and dendritic cells. Brain Behav Immunity 22(1):35–41
Mizoguchi Y et al (2009) Brain-derived neurotrophic factor induces sustained elevation of intracellular Ca2+ in rodent microglia. J Immunol 183(12):7778–7786
Cowell RM et al (2006) Microglial expression of chemokine receptor CCR5 during rat forebrain development and after perinatal hypoxia-ischemia. J Neuroimmunol 173(1–2):155–165
Boddeke E et al (1999) Cultured rat microglia express functional beta-chemokine receptors. J Neuroimmunol 98(2):176–184
Bordey A, Spencer DD (2003) Chemokine modulation of high-conductance Ca2+−sensitive K+ currents in microglia from human hippocampi. Eur J Neurosci 18(10):2893–2898
Rappert A et al (2002) Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglial. J Immunol 168(7):3221–3226
Boddeke E et al (1999) Functional expression of the fractalkine (CX3C) receptor and its regulation by lipopolysaccharide in rat microglia. Eur J Pharmacol 374(2):309–313
Lacy M et al (1995) Expression of the receptors for the C5a anaphylatoxin, Interleukin-8 and Fmlp by human astrocytes and microglia. J Neuroimmunol 61(1):71–78
Butovsky O et al (2007) Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Mol Cell Neurosci 35(3):490–500
Hanisch UK et al (2001) The protein tyrosine kinase inhibits AG126 prevents the massive microglial cytokine induction by pneumococcal cell walls. Eur J Immunol 31(7):2104–2115
Syed MM, Phulwani NK, Kielian T (2007) Tumor necrosis factor-alpha (TNF-alpha) regulates Toll-like receptor 2 (TLR2) expression in microglia. J Neurochem 103(4):1461–1471
Michelucci A et al (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210(1–2):3–12
Stohwasser R et al (2000) Biochemical analysis of proteasomes from mouse microglia: induction of immunoproteasomes by interferon-gamma and lipopolysaccharide. Glia 29(4):355–365
Butovsky O et al (2005) Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci 29(3):381–393
Prinz M et al (2008) Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28(5):675–686
McNamee EN et al (2010) Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1 beta-induced neurotoxicity. Eur J Pharmacol 626(2–3):219–228
Pinteaux E et al (2002) Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem 83(4):754–763
Andre R et al (2005) Regulation of expression of the novel IL-1 receptor family members in the mouse brain. J Neurochem 95(2):324–330
Cunningham ET, Desouza EB (1993) Interleukin-1 receptors in the brain and endocrine tissues. Immunol Today 14(4):171–176
Sawada M, Suzumura A, Marunouchi T (1995) Induction of functional Interleukin-2 receptor in mouse microglia. J Neurochem 64(5):1973–1979
Shimizu E et al (2008) IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia. J Immunol 181(9):6503–6513
Gonzalez P et al (2009) Interleukin-10 and Interleukin-10 receptor-I are upregulated in glial cells after an Excitotoxic injury to the postnatal rat brain. J Neuropathol Exp Neurol 68(4):391–403
Huang Z, Ha GK, Petitto JM (2007) IL-15 and IL-15R alpha gene deletion: Effects on T lymphocyte trafficking and the microglial and neuronal responses to facial nerve axotomy. Neurosci Lett 417(2):160–164
Prinz M, Hanisch UK (1999) Murine microglial cells produce and respond to interleukin-18. J Neurochem 72(5):2215–2218
Bernardino ALF et al (2008) Toll-like receptors: Insights into their possible role in the pathogenesis of Lyme neuroborreliosis. Infect Immun 76(10):4385–4395
Bsibsi M et al (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61(11):1013–1021
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924
Zuiderwijk-Sick EA et al (2007) Differentiation of primary adult microglia alters their response to TLR8-mediated activation but not their capacity as APC. Glia 55(15):1589–1600
Babcock AA et al (2006) Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci 26(49):12826–12837
Chen KQ et al (2006) Activation of toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem 281(6):3651–3659
Ebert S et al (2005) Dose-dependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J Neuroimmunol 159(1–2):87–96
Lehnardt S et al (2006) A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol 177(1):583–592
Town T et al (2006) Microglia recognize double-stranded RNA via TLR3. J Immunol 176(6):3804–3812
Qin LY et al (2005) Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 52(1):78–84
Lehnardt S et al (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22(7):2478–2486
Butchi NB, Du M, Peterson KE (2010) Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia 58(6):650–664
Cervantes JL et al (2012) TLR8: the forgotten relative revindicated. Cell Mol Immunol 9(6):434–438
Dalpke AH et al (2002) Immunostimulatory CpG-DNA activates murine microglia. J Immunol 168(10):4854–4863
Prinz M et al (1999) Microglial activation by components of Gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J Neuropathol Exp Neurol 58(10):1078–1089
Bate C, Boshuizen R, Williams A (2005) Microglial cells kill prion-damaged neurons in vitro by a CD14-dependent process. J Neuroimmunol 170(1–2):62–70
Beschorner R et al (2002) CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol 103(6):541–549
Chattopadhyay N et al (1999) The extracellular calcium-sensing receptor is expressed in rat microglia and modulates an outward K+ channel. J Neurochem 72(5):1915–1922
Moller T et al (1997) Mechanisms of C5a and C3a complement fragment-induced Ca2+ (i) signaling in mouse microglia. J Neurosci 17(2):615–624
Nolte C et al (1996) Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience 73(4):1091–1107
Ilschner S, Nolte C, Kettenmann H (1996) Complement factor C5a and epidermal growth factor trigger the activation of outward potassium currents in cultured murine microglia. Neuroscience 73(4):1109–1120
Wright GJ et al (2003) Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 171(6):3034–3046
Funding
This work was supported by funding from the US Army Medical Research and Material Command (W81XWH-15-1-0303), New Jersey Commission for Brain Injury Research (CBIR17PIL020), and Rutgers Brain Health Institute (BHI-RUN-NJIT-2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Table 1
Microglial receptors and their functional relevance. (DOCX 106 kb)
Rights and permissions
About this article
Cite this article
Younger, D., Murugan, M., Rama Rao, K.V. et al. Microglia Receptors in Animal Models of Traumatic Brain Injury. Mol Neurobiol 56, 5202–5228 (2019). https://doi.org/10.1007/s12035-018-1428-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1428-7