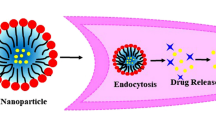
Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous tumor with a poor prognosis and high metastatic potential, resulting in poor clinical outcomes, necessitating investigation to devise effective therapeutic strategies. Multiple studies have substantiated the anti-cancer properties of the naturally occurring flavonoid "Myricetin" in various malignancies. However, the therapeutic application of Myricetin is impeded by its poor water solubility and low oral bioavailability. To overcome this limitation, we aimed to develop nanoemulsion of Myricetin (Myr-NE) and evaluate its advantage over Myricetin alone in TNBC cells. The nanoemulsion was formulated using Capryol 90 (oil), Tween 20 (surfactant), and Transcutol HP (co-surfactant). The optimized nano-formulation underwent an evaluation to determine its size, zeta potential, morphology, stability, drug encapsulation efficiency, and in vitro release properties. The anti-cancer activity of Myr-NE was further studied to examine its distinct impact on intracellular drug uptake, cell-viability, anti-tumor signaling, oxidative stress, clonogenicity, and cell death, compared with Myricetin alone in MDA-MB-231 (TNBC) cells. The in vitro drug release and intracellular drug uptake of Myricetin was significantly increased in Myr-NE formulation as compared to Myricetin alone. Moreover, Myr-NE exhibited significant inhibition of cell proliferation, clonogenicity, and increased apoptosis with ~ 2.5-fold lower IC50 as compared to Myricetin. Mechanistic investigation revealed that nanoemulsion augmented the anti-cancer efficacy of Myricetin, most likely by inhibiting the PI3K/AKT/mTOR pathway, eventually leading to enhanced cell death in TNBC cells. The study provides substantial experimental evidence to support the notion that the Myr-NE formulation has the potential to be an effective therapeutic drug for TNBC treatment.
Graphical Abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Khan MA, Jain VK, Rizwanullah M, Ahmad J, Jain K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov Today. 2019;24(11):2181–91. https://doi.org/10.1016/j.drudis.2019.09.001.
Pawar A, Prabhu P. Nanosoldiers: a promising strategy to combat triple negative breast cancer. Biomed Pharmacother. 2019;110:319–41. https://doi.org/10.1016/j.biopha.2018.11.122.
Beniey M, Hubert A, Haque T, Cotte AK, Béchir N, Zhang X, Tran-Thanh D, Hassan S. Sequential targeting of PARP with carboplatin inhibits primary tumour growth and distant metastasis in triple-negative breast cancer. Br J Cancer. 2023;20:1–2. https://doi.org/10.1038/s41416-023-02226-w.
Yue X, Li M, Chen D, Xu Z, Sun S. UNBS5162 induces growth inhibition and apoptosis via inhibiting PI3K/AKT/mTOR pathway in triple negative breast cancer MDA-MB-231 cells. Exp Ther Med. 2018;16(5):3921–8. https://doi.org/10.3892/etm.2018.6675.
Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:1–3. https://doi.org/10.1186/s13058-020-01296-5.
Dang Y, Lin G, Xie Y, Duan J, Ma P, Li G, Ji G. Quantitative determination of Myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res. 2014;64(10):516–22. https://doi.org/10.1055/s-0033-1363220.
Guo RX, Fu X, Chen J, Zhou L, Chen G. Preparation and characterization of microemulsions of Myricetin for improving its anti-proliferative and antioxidative activities and oral bioavailability. J Agric Food Chem. 2016;64(32):6286–94. https://doi.org/10.1021/acs.jafc.6b02184.
Zhu ML, Zhang PM, Jiang M, Yu SW, Wang L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement Med Therapies. 2020;20:1–9. https://doi.org/10.1186/s12906-020-02965-w.
Cui YH, Chen J, Xu T, Tian HL. Structure-based grafting and identification of kinase–inhibitors to target mTOR signaling pathway as potential therapeutics for glioblastoma. Comput Biol Chem. 2015;54:57–65. https://doi.org/10.1016/j.compbiolchem.2015.01.001.
Alhalmi A, Beg S, Kohli K, Waris M, Singh T. Nanotechnology based approach for hepatocellular carcinoma targeting. Curr Drug Targets. 2021;22(7):779–92. https://doi.org/10.2174/1389450121999201209194524.
Phillips PA, Sangwan V, Borja-Cacho D, Dudeja V, Vickers SM, Saluja AK. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011;308(2):181–8. https://doi.org/10.1016/j.canlet.2011.05.002.
Paplomata, E. and O'Regan, R., 2014. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Therapeutic advances in medical oncology, 6(4), pp.154–166. https:// doi: https://doi.org/10.1177/1758834014530023.
Glaviano A, Foo AS, Lam HY, Yap KC, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, Kulke MH. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22(1):1–37. https://doi.org/10.1186/s12943-023-01827-6.
Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: aAt the bench and bedside. In: Seminars in cancer biology, vol. 59. New York: Academic Press; 2019. p. 125–32.
He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):425. https://doi.org/10.1038/s41392-021-00828-5.
Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol. 2019;30(7):1051–60. https://doi.org/10.1093/annonc/mdz133.
Dou JW, Shang RG, Lei XQ, Li KL, Guo ZZ, Ye K, Huang Q. Total saponins of Bolbostemma paniculatum (maxim.) Franquet exert anti-tumor activity against MDA-MB-231 human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. BMC Complement Alternat Med. 2019;19:1–9. https://doi.org/10.1186/s12906-019-2708-0.
Guo Y, Pei X. Tetrandrine-induced autophagy in MDA-MB-231 triple-negative breast cancer cell through the inhibition of PI3K/AKT/mTOR signaling. Evid-Based Complement Alternat Med. 2019. https://doi.org/10.1155/2019/7517431.
Man N, Wang Q, Li H, Adu-Frimpong M, Sun C, Zhang K, Xu X. Improved oral bioavailability of myricitrin by liquid self-microemulsifying drug delivery systems. J Drug Deliv Sci Technol. 2019;52:597–606. https://doi.org/10.1016/j.jddst.2019.05.003.
Vatsraj S, Chauhan K, Pathak H. Formulation of a novel nanoemulsion system for enhanced solubility of a sparingly water soluble antibiotic, clarithromycin. J Nanosci. 2014. https://doi.org/10.1155/2014/2682938.
Tawfik NM, Teiama MS, Iskandar SS, Osman A, Hammad SF. A novel nanoemulsion formula for an improved delivery of a thalidomide analogue to triple-negative breast cancer; synthesis, formulation, characterization and molecular studies. Int J Nanomed. 2023. https://doi.org/10.2147/IJN.S385166.
Kim B, Hebert JM, Liu D, Auguste DT. A lipid targeting, pH-responsive nanoemulsion encapsulating a DNA intercalating agent and HDAC inhibitor reduces TNBC tumor burden. Adv Therapeutics. 2021;4(3):2000211. https://doi.org/10.1002/adtp.202000211.
Song X, Zhang Y, Zuo R, Zhang J, Lin M, Wang J, Hu S, Ji H, Peng L, Lv Y, Gao X. Repurposing maduramicin as a novel anti-cancer and anti-metastasis agent for triple-negative breast cancer as enhanced by nanoemulsion. Int J Pharm. 2022;625:122091. https://doi.org/10.1016/j.ijpharm.2022.122091.
Ombredane AS, Silva LR, Araujo VH, Costa PL, Silva LC, Sampaio MC, Lima MC, Junior VFV, Vieira IJ, Azevedo RB, Joanitti GA. Pequi oil (Caryocar brasilense Cambess.) nanoemulsion alters cell proliferation and damages key organelles in triple-negative breast cancer cells in vitro. Biomed Pharmacother. 2022;153:113348. https://doi.org/10.1016/j.biopha.2022.113348.
Kim B, Pena CD, Auguste DT. Targeted lipid nanoemulsions encapsulating epigenetic drugs exhibit selective cytotoxicity on CDH1–/FOXM1+ triple negative breast cancer cells. Mol Pharm. 2019;16(5):1813–26. https://doi.org/10.1021/acs.molpharmaceut.8b01065.
Saraiva SM, Gutiérrez-Lovera C, Martínez-Val J, Lores S, Bouzo BL, Díez-Villares S, Alijas S, Pensado-López A, Vázquez-Ríos AJ, Sánchez L, de la Fuente M. Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci Rep. 2021;11(1):9873. https://doi.org/10.1038/s41598-021-87968-4.
Zanesco-Fontes I, Silva ACL, da Silva PB, Duarte JL, Di Filippo LD, Chorilli M, Cominetti MR, Martin ACBM. [10]-Gingerol-loaded nanoemulsion and its biological effects on triple-negative breast cancer cells. AAPS PharmSciTech. 2021;22(5):157. https://doi.org/10.1208/s12249-021-02006-w.
Chime SA, Kenechukwu FC, Attama AA. Nanoemulsions-advances in formulation, characterization and applications in drug delivery. Appl Nanotechnol Drug Deliv. 2014;3:77–126. https://doi.org/10.5772/58673.
Sharma S, Sahni JK, Ali J, Baboota S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin–pharmacodynamic and antioxidant studies. Drug Deliv. 2015;22(4):541–51. https://doi.org/10.3109/10717544.2014.893382.
Khan R, Mirza MA, Aqil M, Hassan N, Zakir F, Ansari MJ, Iqbal Z. A pharmaco-technical investigation of thymoquinone and peat-sourced fulvic acid nanoemulgel: a combination therapy. Gels. 2022;8(11):733. https://doi.org/10.3390/gels8110733.
Mohammadifar M, Aarabi MH, Aghighi F, Kazemi M, Vakili Z, Memarzadeh MR, Talaei SA. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: behavioral, biochemical, and histopathological evidence. BMC Complement Med Ther. 2021;21(1):1–2. https://doi.org/10.1186/s12906-021-03236-y.
Lima TS, Silva MF, Nunes XP, Colombo AV, Oliveira HP, Goto PL, Blanzat M, Piva HL, Tedesco AC, Siqueira-Moura MP. Cineole-containing nanoemulsion: development, stability, and antibacterial activity. Chem Phys Lipids. 2021;239:105113. https://doi.org/10.1016/j.chemphyslip.2021.105113.
Gokhale JP, Mahajan HS, Surana SJ. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: in vivo and in vitro studies. Biomed Pharmacother. 2019;112:108622–8. https://doi.org/10.1016/j.biopha.2019.108622.
Ali HH, Hussein AA. Oral nanoemulsions of candesartan cilexetil: formulation, characterization and in vitro drug release studies. Aaps Open. 2017;3(1):1–16. https://doi.org/10.1186/s41120-017-0016-7.
Ahmed S, Gull A, Alam M, Aqil M, Sultana Y. Ultrasonically tailored, chemically engineered and “QbD” enabled fabrication of agomelatine nanoemulsion; optimization, characterization, ex-vivo permeation and stability study. Ultrason Sonochem. 2018;1(41):213–26. https://doi.org/10.1016/j.ultsonch.2017.09.042.
Gull A, Ahmed S, Ahmad FJ, Nagaich U, Chandra A. Effect of polyherbal microemulsion on Staphylococcus epidermidis: formulation development, CCD based optimization, characterization, and antibacterial activity by scanning electron microscopy. J Drug Deliv Sci Technol. 2020;57:101641. https://doi.org/10.1016/j.jddst.2020.101641.
Laxmi M, Bhardwaj A, Mehta S, Mehta A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells Nanomed Biotechnol. 2015;43(5):334–44. https://doi.org/10.3109/21691401.2014.887018.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–6. https://doi.org/10.1038/nprot.2006.179.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/nejmoa1809615.
Rai Y, Kumari N, Singh S, Kalra N, Soni R, Bhatt AN. Mild mitochondrial uncoupling protects from ionizing radiation induced cell death by attenuating oxidative stress and mitochondrial damage. Biochim Biophys Acta. 2021;1862(1):148325. https://doi.org/10.1016/j.bbabio.2020.148325.
Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part I: Preformulation studies, formulation design and physicochemical evaluation. Int J Pharm. 2012;431(1–2):138–48. https://doi.org/10.1016/j.ijpharm.2011.12.040.
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, Talegaonkar S. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10:69–76. https://doi.org/10.1208/s12249-008-9178-x.
Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):1–28. https://doi.org/10.1186/s12943-019-0954-x.
Phillips PA, Sangwan V, Borja-Cacho D, Dudeja V, Vickers SM, Saluja AK. Myricetin induces pancreatic cancecr cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011;308(2):181–8. https://doi.org/10.1016/j.canlet.2011.05.002.
Han SH, Lee JH, Woo JS, Jung GH, Jung SH, Han EJ, Kim B, Cho SD, Nam JS, Che JH, Jung JY. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon. 2022. https://doi.org/10.1016/j.heliyon.2022.e09309.
Jaglanian A, Tsiani E. Rosemary extract inhibits proliferation, survival, Akt, and mTOR signaling in triple-negative breast cancer cells. Int J Mol Sci. 2020;21(3):810. https://doi.org/10.3390/ijms21030810.
Jiang M, Zhu M, Wang L, Yu S. Anti-tumor effects and associated molecular mechanisms of Myricetin. Biomed Pharmacother. 2019;120: 109506. https://doi.org/10.1016/j.biopha.2019.109506.
Knickle A, Rasmussen A, Hoskin DW. Myricetin induces apoptosis mediated by oxidative stress in 4T1 and E0771 mammary cancer cells. Mol Cell Toxicol. 2020;16:283–9. https://doi.org/10.1016/j.fct.2018.05.005.
Knickle A, Fernando W, Greenshields AL, Rupasinghe HV, Hoskin DW. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol. 2018;118:154–67. https://doi.org/10.1016/j.fct.2018.05.005.
Rhee J, Han SW, Oh DY, Kim JH, Im SA, Han W, Kim TY. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8(1):1–8. https://doi.org/10.1186/1471-2407-8-307.
Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25:S142–50. https://doi.org/10.3747/co.25.3954.
Costa RL, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treatm. 2018;169:397–406. https://doi.org/10.1007/s10549-018-4697-y.
Singh S, Sharma B, Kanwar SS, Kumar A. Lead phytochemicals for anti-cancer drug development. Front Plant Sci. 2016;7:1667. https://doi.org/10.3389/fpls.2016.01667.
Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):2712. https://doi.org/10.1200/JCO.2008.20.6235.
Ko CH, Shen SC, Hsu CS, Chen YC. Mitochondrial-dependent, reactive oxygen species-independent apoptosis by Myricetin: roles of protein kinase C, cytochrome c, and caspase cascade. Biochem Pharmacol. 2005;69(6):913–27. https://doi.org/10.1016/j.bcp.2004.12.005.
Shih YW, Wu PF, Lee YC, Shi MD, Chiang TA. Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: possible mediation by blocking the ERK signaling pathway. J Agric Food Chem. 2009;57(9):3490–9. https://doi.org/10.1021/jf900124r.
Chrastina A, Welsh J, Borgström P, Baron VT. Propylene glycol caprylate-based nanoemulsion formulation of plumbagin: development and characterization of anti-cancer activity. BioMed Res Int. 2022. https://doi.org/10.1155/2022/3549061.
Lee WD, Liang YJ, Chen BH. Effects of tanshinone nanoemulsion and extract on inhibition of lung cancer cells A549. Nanotechnology. 2016;27(49):495101. https://doi.org/10.1088/0957-4484/27/49/495101.
Handa M, Ujjwal RR, Vasdev N, Flora SJ, Shukla R. Optimization of surfactant-and cosurfactantaided pine oil nanoemulsions by isothermal low-energy methods for anticholinesterase activity. ACS Omega. 2020;6(1):559–68.
Gutiérrez JM, González C, Maestro A, Solè IM, Pey CM, Nolla J. Nanoemulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13(4):245–51.
van Jaarsveld MT, Deng D, Ordoñez-Rueda D, Paulsen M, Wiemer EA, Zi Z. Cell-type-specific role of CHK2 in mediating DNA damage-induced G2 cell cycle arrest. Oncogenesis. 2020;9(3):35. https://doi.org/10.1038/s41389-020-0219-y.
Sharma P, Khan MA, Najmi AK, Chaturvedi S, Akhtar M. Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med Oncol. 2022;39(12):248. https://doi.org/10.1007/s12032-022-01856-z.
Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anti-cancer Res. 2014;34(2):701–6.
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, Li W, Hu J, Lu C, Liu Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. https://doi.org/10.1038/s41419-020-02998-6.
Acknowledgements
PS acknowledges the Indian Council of Medical Research for fellowship support [Grant number 45/24/20202-Nan-BMS]. We also acknowledge Ms. Namita Kalra for helping with the data acquisition on the flow cytometer.
Funding
This work was supported by the Indian Council of Medical Research [Grant number 45/24/20202-Nan-BMS]. Author PS has received fellowship support from the Indian Council of Medical Research.
Author information
Authors and Affiliations
Contributions
PS: Conceptualization, investigation, methodology, data curation, Writing—original draft. SC: Formal analysis, writing—review & editing. MK: Formal analysis, writing—review & editing. YR: In vitro investigation, review & editing. AB: In vitro data analysis, review & editing. MA: Writing—review & editing. AM: Formal analysis—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, P., Chaturvedi, S., Khan, M.A. et al. Nanoemulsion potentiates the anti-cancer activity of Myricetin by effective inhibition of PI3K/AKT/mTOR pathway in triple-negative breast cancer cells. Med Oncol 41, 56 (2024). https://doi.org/10.1007/s12032-023-02274-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02274-5