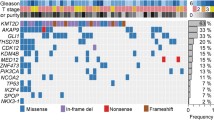
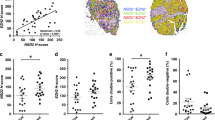
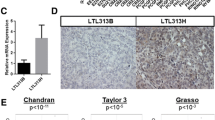
Abstract
Prostate cancer (PCa) is one of the most common malignant tumors that exhibit both chemoresistance and recurrence. SUV39H2 is highly expressed in many types of human tumors, but its role in the development and progression of PCa has never been clarified. The aim of this study is to elucidate the role of SUV39H2 in the development and progression of PCa, its association with the AKT/FOXO signaling pathway, and its potential implications for PCa diagnosis and treatment. SUV39H2 expression was analyzed in The Cancer Genome Atlas (TCGA) and genotype tissue expression pan-cancer data. The TCGA database was evaluated for SUV39H2 enrichment and its correlation to immune cell infiltration. SUV39H2 levels in PCa tissues and control tissues were determined in 30 patients using qPCR and IHC. Clinical relevance was assessed via The Cancer Genome Atlas (TCGA). In vitro assessments including colony formation assays, Western Blot analysis, CCK-8 assays, and flow cytometry were utilized to establish SUV39H2’s contribution to PCa cell growth. The influence of SUV39H2 on PC3 and DU145 cell proliferation was assessed through a cell line-derived xenograft model. Sphere formation assays and qPCR were employed to delineate SUV39H2’s role in PCa stemness and chemosensitivity. In vitro macrophage polarization assays provided insights into SUV39H2’s association with M2 macrophages, while enrichment analysis shed light on its role in FOXO signaling. PCa tissues expressed higher levels of SUV39H2 than normal tissues. By knocking down SUV39H2, PCa cells were made more chemosensitive to docetaxel and cell proliferation and stemness were inhibited. Additionally, SUV39H2 knockdown significantly inhibited in vivo PCa cell growth and inhibited the polarization of macrophages. Furthermore, SUV39H2 was found to regulate AKT/FOXO signaling by increasing Akt and FOXO3a phosphorylation. Our findings highlight SUV39H2’s role in PCa cell apoptosis and chemosensitivity mainly by regulating the AKT/FOXO signaling pathway and suggest that SUV39H2 could be a potential target for PCa diagnosis and treatment.








Similar content being viewed by others
Data availability
All data for this study are included in this article.
References
Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32:1105–40.
Zhao K, Li D, Xu W, Ding J, Jiang W, Li M, et al. Targeted hydroxyethyl starch prodrug for inhibiting the growth and metastasis of prostate cancer. Biomaterials. 2017;116:82–94.
Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harbor Persp Med. 2018;8:a030361.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA: Cancer J Clin. 2020;70:145–64.
Hariri L, Rehman A. Estradiol. Treasure Island: Stat-Pearls Publishing LLC; 2022.
Peng Y, Dong S, Yang Z, Song Y, Ding J, Hou D, et al. Identification of docetaxel-related biomarkers for prostate cancer. Andrologia. 2021;53:e14079.
Pérez G, López-Moncada F, Indo S, Torres MJ, Castellón EA, Contreras HR. Knockdown of ZEB1 reverses cancer stem cell properties in prostate cancer cells. Oncol Rep. 2021;45:1–12.
Püschel J, Dubrovska A, Gorodetska I. The multifaceted role of aldehyde dehydrogenases in prostate cancer stem cells. Cancers. 2021;13:4703.
Schwarz FM, Schniewind I, Besso MJ, Lange S, Linge A, Patil SG, et al. Plasticity within aldehyde dehydrogenase-positive cells determines prostate cancer radiosensitivity. Mol Cancer Res. 2022;20:794–809.
Lee SI, Roney MSI, Park JH, Baek JY, Park J, Kim SK, et al. Dopamine receptor antagonists induce differentiation of PC-3 human prostate cancer cell-derived cancer stem cell-like cells. Prostate. 2019;79:720–31.
Ren W, Wang D, Li C, Shu T, Zhang W, Fu X. Capn4 expression is modulated by microRNA-520b and exerts an oncogenic role in prostate cancer cells by promoting Wnt/β-catenin signaling. Biomed Pharmacother. 2018;108:467–75.
Xu P, Cai F, Liu X, Guo L. LKB1 suppresses proliferation and invasion of prostate cancer through hedgehog signaling pathway. Int J Clin Exp Pathol. 2014;7:8480.
Xiao L, Peng H, Yan M, Chen S. Silencing ACTG1 expression induces prostate cancer epithelial mesenchymal transition through MAPK/ERK signaling pathway. DNA Cell Biol. 2021;40:1445–55.
Lai W, Zhu W, Xiao C, Li X, Wang Y, Han Y, et al. HJURP promotes proliferation in prostate cancer cells through increasing CDKN1A degradation via the GSK3β/JNK signaling pathway. Cell Death Dis. 2021;12:583.
Yang J-Y, Hung M-C. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–7.
Haflidadóttir BS, Larne O, Martin M, Persson M, Edsjö A, Bjartell A, et al. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS ONE. 2013;8:e72400.
Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–55.
Yan Y, Huang H. Interplay among PI3K/AKT, PTEN/FOXO and AR signaling in prostate cancer. Adv Exp Med Biol. 2019;1210:319–31.
Wu Y, Sarkissyan M, Vadgama JV. Epigenetics in breast and prostate cancer. In: Cancer epigenetics: risk assessment, diagnosis, treatment, and prognosis. Springer; 2015. p. 425–66.
Vieira FQ, Costa-Pinheiro P, Ramalho-Carvalho J, Pereira A, Menezes FD, Antunes L, et al. Deregulated expression of selected histone methylases and demethylases in prostate carcinoma. Endocr Relat Cancer. 2014;21:51–61.
Li B, Zheng Y, Yang L. The oncogenic potential of SUV39H2: a comprehensive and perspective view. J Cancer. 2019;10:721.
Piao L, Nakakido M, Suzuki T, Dohmae N, Nakamura Y, Hamamoto R. Automethylation of SUV39H2, an oncogenic histone lysine methyltransferase, regulates its binding affinity to substrate proteins. Oncotarget. 2016;7:22846.
Askew EB, Bai S, Parris AB, Minges JT, Wilson EM. Androgen receptor regulation by histone methyltransferase suppressor of variegation 3–9 homolog 2 and Melanoma antigen-A11. Mol Cell Endocrinol. 2017;443:42–51.
Baratchian M, Tiwari R, Khalighi S, Chakravarthy A, Yuan W, Berk M, et al. H3K9 methylation drives resistance to androgen receptor–antagonist therapy in prostate cancer. Proc Natl Acad Sci. 2022;119:e2114324119.
Hung S-Y, Lin H-H, Yeh K-T, Chang J-G. Histone-modifying genes as biomarkers in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:2496.
Miao Y, Liu G, Liu L. Histone methyltransferase SUV39H2 regulates LSD1-dependent CDH1 expression and promotes epithelial mesenchymal transition of osteosarcoma. Cancer Cell Int. 2021;21:1–12.
Reyes DA, Sarría VMS, Salazar-Viedma M, D’Afonseca V. Histone methyltransferases useful in gastric cancer research. Cancer Informatics. 2021;20:11769351211039862.
Shuai W, Wu J, Chen S, Liu R, Ye Z, Kuang C, et al. SUV39H2 promotes colorectal cancer proliferation and metastasis via tri-methylation of the SLIT1 promoter. Cancer Lett. 2018;422:56–69.
Yoon K-A, Hwangbo B, Kim I-J, Park S, Kim HS, Kee HJ, et al. Novel polymorphisms in the SUV39H2 histone methyltransferase and the risk of lung cancer. Carcinogenesis. 2006;27:2217–22.
Zheng Y, Li B, Wang J, Xiong Y, Wang K, Qi Y, et al. Identification of SUV39H2 as a potential oncogene in lung adenocarcinoma. Clin Epigenetics. 2018;10:1–11.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8.
Hu Y, Zhang H, Xie N, Liu D, Jiang Y, Liu Z, et al. Bcl-3 promotes TNF-induced hepatocyte apoptosis by regulating the deubiquitination of RIP1. Cell Death Differ. 2022;29:1176–86.
Lu X, Yang F, Chen D, Zhao Q, Chen D, Ping H, et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int J Biol Sci. 2020;16:1121.
Ye D, Liu H, Zhao G, Chen A, Jiang Y, Hu Y, et al. LncGMDS-AS1 promotes the tumorigenesis of colorectal cancer through HuR-STAT3/Wnt axis. Cell Death Dis. 2023;14:165.
Jia G, Wang X, Wu W, Zhang Y, Chen S, Zhao J, et al. LXA4 enhances prostate cancer progression by facilitating M2 macrophage polarization via inhibition of METTL3. Int Immunopharmacol. 2022;107:108586.
Tsirmoula S, Dimas K, Hatziapostolou M, Lamprou M, Ravazoula P, Papadimitriou E. Implications of pleiotrophin in human PC 3 prostate cancer cell growth in vivo. Cancer Sci. 2012;103:1826–32.
Zhang K, Waxman DJ. PC3 prostate tumor-initiating cells with molecular profile FAM65Bhigh/MFI2low/LEF1low increase tumor angiogenesis. Mol Cancer. 2010;9:1–13.
Jaworska D, Król W, Szliszka E. Prostate cancer stem cells: research advances. Int J Mol Sci. 2015;16:27433–49.
Skvortsov S, Skvortsova I-I, Tang DG, Dubrovska A. Concise review: prostate cancer stem cells: current understanding. Stem Cells. 2018;36:1457–74.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090.
Angeles AK, Heckmann D, Flosdorf N, Duensing S, Sültmann H. The ERG-regulated LINC00920 promotes prostate cancer cell survival via the 14-3-3ϵ–FOXO pathway. Mol Cancer Res. 2020;18:1545–59.
Wang S, Xu G, Chao F, Zhang C, Han D, Chen G. HNRNPC promotes proliferation, metastasis and predicts prognosis in prostate cancer. Cancer Manag Res. 2021;13:7263–76.
Holliday R. Epigenetics: an overview. Dev Genet. 1994;15:453–7.
García-Cao M, O’Sullivan R, Peters AH, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet. 2004;36:94–9.
Nielsen SJ, Schneider R, Bauer U-M, Bannister AJ, Morrison A, O’Carroll D, et al. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412:561–5.
Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–6.
Baylin SB, Jones PA. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer. 2011;11:726–34.
Dobosy JR, Roberts JLW, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–31.
Perry AS, Watson RWG, Lawler M, Hollywood D. The epigenome as a therapeutic target in prostate cancer. Nat Rev Urol. 2010;7:668–80.
Leão R, Domingos C, Figueiredo A, Hamilton R, Tabori U, Castelo-Branco P. Cancer stem cells in prostate cancer: implications for targeted therapy. Urol Int. 2017;99:125–36.
Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81.
Obeng E. Apoptosis (programmed cell death) and its signals—a review. Braz J Biol. 2020;81:1133–43.
Li X, Liu K, Zhou W, Jiang Z. MiR-155 targeting FoxO3a regulates oral cancer cell proliferation, apoptosis, and DDP resistance through targeting FoxO3a. Cancer Biomark. 2020;27:105–11.
Emre Kızıl H, Gür C, Ayna A, Darendelioğlu E, Küçükler S, Sağ S. Contribution of oxidative stress, apoptosis, endoplasmic reticulum stress and autophagy pathways to the ameliorative effects of hesperidin in NaF-induced testicular toxicity. Chem Biodivers. 2023;20:e202200982.
Kızıl HE, Caglayan C, Darendelioğlu E, Ayna A, Gür C, Kandemir FM, et al. Morin ameliorates methotrexate-induced hepatotoxicity via targeting Nrf2/HO-1 and Bax/Bcl2/caspase-3 signaling pathways. Mol Biol Rep. 2023;50:3479–88.
Liu Y, Wang Y, Li X, Jia Y, Wang J, Ao X. FOXO3a in cancer drug resistance. Cancer Lett. 2022;540:215724.
Varışlı B, Darendelioğlu E, Caglayan C, Kandemir FM, Ayna A, Genç A, et al. Hesperidin attenuates oxidative stress, inflammation, apoptosis, and cardiac dysfunction in sodium fluoride-Induced cardiotoxicity in rats. Cardiovasc Toxicol. 2022;22:727–35.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86.
Crumbaker M, Khoja L, Joshua AM. AR signaling and the PI3K pathway in prostate cancer. Cancers. 2017;9:34.
Pearson HB, Li J, Meniel VS, Fennell CM, Waring P, Montgomery KG, et al. Identification of Pik3ca mutation as a genetic driver of prostate cancer that cooperates with Pten loss to accelerate progression and castration-resistant growth. Cancer Discov. 2018;8:764–79.
Acknowledgements
Not applicable.
Funding
This work was supported by Shenzhen Fundamental Research Program (Grant No. JCYJ20190808095615389).
Author information
Authors and Affiliations
Contributions
Conception and design: DS, XC, and LW; Administrative support: JG, XC, and WL; Provision of study materials or patients: XC; Collection and assembly of data: DS and XC; Data analysis and interpretation: SL, QL, QL, and YC; Manuscript writing: XC, YS, SY, and AL; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Ethical approval
PCa and paracancerous tissue specimens were taken after informed and written consent and the study was approved by the Ethics Committee of Shenzhen Hospital, Southern Medical University (Shenzhen, China). All animal procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publications No.8023, revised 1978) and approved by the Institutional Biomedical Research Ethics Committee of Qingyuan People’s Hospital, and the study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1
The Relationship between SUV39H2 Expression Level and Prognosis of Tumor Patients. a Forest plot of hazard ratios of SUV39H2 in 27 types of tumors. b Univariate and Multivariate Cox regression analyses regarding OS in prostate cancer. c The curve of risk score and Survival status of the patients. A higher risk score is associated with more deaths. Heatmap of the expression profiles of SUV39H2 prognostic genes in low- and high-risk group. d Kaplan–Meier survival analysis of SUV39H2. e Time-dependent ROC analysis
Supplementary file1 (TIF 104787 KB)
Supplementary Figure 2
Pan-cancer analysis of the correlation between SUV39H2 expression and immune checkpoint genes and immune regulators TMB and MSI. a Pan-cancer analysis of the correlation between SUV39H2 expression and immune checkpoint genes. b and c. Pan-cancer analysis of the correlation between SUV39H2 expression and immunomodulators TMB and MSI. *p <0.05, **p <0.01, and ***p <0.001
Supplementary file2 (PDF 466 KB)
Supplementary Figure 3 A–D
The correlation of SUV39H2 with infiltration of immune cells in the TCGA database
Supplementary file3 (TIF 5791 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, D., Guo, J., Liang, W. et al. Histone methyltransferase SUV39H2 regulates apoptosis and chemosensitivity in prostate cancer through AKT/FOXO signaling pathway. Med Oncol 41, 44 (2024). https://doi.org/10.1007/s12032-023-02252-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02252-x