Abstract
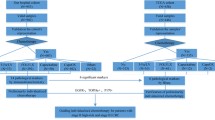
Although some studies in China have suggested Huachansu (HCS) combined with chemotherapy is effective in the treatment of various cancers, there are few studies on colorectal cancer (CRC), especially in postoperative adjuvant chemotherapy. The aim of this study was to test the hypothesis that HCS combined with adjuvant chemotherapy would improve survival probability in resected CRC patients. This was a prospective, open-label, randomized phase II study. Patients with stage III or high-risk stage II resected CRC were randomly assigned to the chemotherapy and HCS + chemotherapy groups. The Chemotherapy group was treated with the FOLFOX regimen for ≥ 6 cycles or the CAPEOX regimen for ≥ 4 cycles. The HCS + chemotherapy group was treated with HCS on the basis of the chemotherapy group. The primary endpoint was 3-year disease-free survival (DFS), and the secondary endpoints were 3-year overall survival (OS) and toxicity. A total of 250 patients were included in this study (126 chemotherapy, 124 HCS + chemotherapy). There were significant differences in 3-year DFS between the two groups (median 28.7 vs. 31.6 months, respectively; P = 0.027), but no significant differences in 3-year OS between the two groups (median 32.7 vs. 34 months, respectively; P = 0.146). No patients experienced grade four adverse events, and the rates of leukopenia, neutropenia, and diarrhea in the HCS + chemotherapy group were lower than that those in the chemotherapy group. HCS combined with adjuvant chemotherapy after radical resection for patients with stage III or high-risk stage II CRC was demonstrated to be an effective and feasible treatment.


Similar content being viewed by others
Data availability
The corresponding author can provide the datasets used or analyzed in the current study upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71(3):209–49.
Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics. Chin Med J (Engl). 2021;134(7):783–91.
Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324–32.
Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol. 2007;25(21):3061–8.
Tiselius C, Gunnarsson U, Smedh K, et al. Patients with rectal cancer receiving adjuvant chemotherapy have an increased survival: a population-based longitudinal study. Ann Oncol. 2013;24(1):160–5.
Schmoll HJ, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15(13):1481–92.
Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5.
Dhakal P, Wichman CS, Pozehl B, et al. Preferences of adults with cancer for systemic cancer treatment: do preferences differ based on age? Future Oncol. 2022;18(3):311–21.
Chionh F, Lau D, Yeung Y, et al. Oral versus intravenous fluoropyrimidines for colorectal cancer. Cochrane Database Syst Rev. 2017;7(7):D8398.
Zhan X, Wu H, Wu H, et al. Metabolites from Bufo gargarizans (Cantor, 1842): a review of traditional uses, pharmacological activity, toxicity and quality control. J Ethnopharmacol. 2020;246:112178.
Li R, Wu H, Wang M, et al. An integrated strategy to delineate the chemical and dynamic metabolic profile of Huachansu tablets in rat plasma based on UPLC-ESI-QTOF/MS(E). J Pharm Biomed Anal. 2022;218: 114866.
Peng C, Chen J, Cui W, et al. Comparative efficacy of various CHIs combined with western medicine for non-small cell lung cancer: a Bayesian network meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:1037620.
Wu J, Zhang D, Ni M, et al. Effectiveness of Huachansu injection combined with chemotherapy for treatment of gastric cancer in China: a systematic review and meta-analysis. J Tradit Chin Med. 2020;40(5):749–57.
Zhang D, Ni M, Wu J, et al. The optimal Chinese herbal injections for use with radiotherapy to treat esophageal cancer: a systematic review and bayesian network meta-analysis. Front Pharmacol. 2018;9:1470.
Tang M, He B, Zhai J, et al. Oral Chinese patent medicine combined with oxaliplatin-based chemotherapy regimen for the treatment of colorectal cancer: a network meta-analysis. Integr Cancer Ther. 2021;20:1543373305. https://doi.org/10.1177/15347354211058169.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14. https://doi.org/10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
Lu XM, Zheng J, Zhu YJ. [Effect of Chinese materia medica combined chemotherapy on the survivals of stage II and III colorectal cancer]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(9):1166–70.
Sun J, Jiang T, Qiu Z, et al. Short-term and medium-term clinical outcomes of laparoscopic-assisted and open surgery for colorectal cancer: a single center retrospective case-control study. BMC Gastroenterol. 2011;11:85.
Tao F. Effect of Huachasu on prevention and treatment of postoperative recurrence and metastasis of colorectal cancer. Guangdong Med J. 2013;34(24):3808–9.
Sun Q, He M, Zhang M, et al. Traditional Chinese medicine and colorectal cancer: implications for drug discovery. Front Pharmacol. 2021;12: 685002.
Qi F, Li A, Inagaki Y, et al. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int Immunopharmacol. 2011;11(3):342–9.
Wei WL, Hou JJ, Wang X, et al. Venenum bufonis: an overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J Ethnopharmacol. 2019;237:215–35.
Shi XK, Cheng C, Guo LS, et al. Clinical observation of Huachansu in preventing postoperative recurrence and metastasis of colorectal cancer. Shenzhen J Integr Tradit Chin West Med. 2016;26(11):72–3.
Meng Z, Garrett CR, Shen Y, et al. Prospective randomised evaluation of traditional Chinese medicine combined with chemotherapy: a randomised phase II study of wild toad extract plus gemcitabine in patients with advanced pancreatic adenocarcinomas. Br J Cancer. 2012;107(3):411–6.
Meng Z, Yang P, Shen Y, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115(22):5309–18.
Tan X, Liang X, Xi J, et al. Clinical efficacy and safety of Huachansu injection combination with platinum-based chemotherapy for advanced non-small cell lung cancer:a systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100(36): e27161.
McQuade RM, Stojanovska V, Abalo R, et al. Chemotherapy-induced constipation and diarrhea: pathophysiology, current and emerging treatments. Front Pharmacol. 2016;7:414.
Qu L, Tan W, Yang J, et al. Combination compositions composed of l-glutamine and Si-Jun-Zi-tang might be a preferable choice for 5-fluorouracil-induced intestinal mucositis: an exploration in a mouse model. Front Pharmacol. 2020;11:918.
Tarricone R, Abu KD, Nyanzi-Wakholi B, et al. A systematic literature review of the economic implications of chemotherapy-induced diarrhea and its impact on quality of life. Crit Rev Oncol Hematol. 2016;99:37–48.
Maroun JA, Anthony LB, Blais N, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian working group on chemotherapy-induced diarrhea. Curr Oncol. 2007;14(1):13–20.
Bai Y, Wang S, Xu W, et al. Cinobufacini ameliorates experimental colitis via modulating the composition of gut microbiota. PLoS ONE. 2019;14(9): e223231.
Burzykowski T, Buyse M, Yothers G, et al. Exploring and validating surrogate endpoints in colorectal cancer. Lifetime Data Anal. 2008;14(1):54–64.
Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19(4):562–78.
Funding
This study was supported by the National Natural Science Foundation of China (81973625), the key medical specialty plan of Shanghai (ZK2019B18), the construction project of clinical special diseases of health system in Putuo District, Shanghai (2019tszk01), and the independent innovation research project of health system in Putuo District, Shanghai (2013ptkw002).
Author information
Authors and Affiliations
Contributions
SL: Conceptualization (equal); investigation (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). DS: Investigation (equal); methodology (equal); writing—review and editing (equal). QZ: Investigation (equal); writing—review and editing (equal). SW: Investigation (equal); writing—review and editing (equal). LM: Investigation (equal); writing—review and editing (equal). JY: Investigation (equal); writing—review and editing (equal). YL: Investigation (equal); writing—review and editing (equal). WL: Methodology (equal); writing—review and editing (equal). CC: Investigation (equal); writing—review and editing (equal). PY: Investigation (equal); writing—review and editing (equal). TC: Conceptualization (equal); data curation (equal); funding acquisition (equal); investigation (equal); supervision (equal); writing—review and editing (equal). JW: Conceptualization (equal); data curation (equal); funding acquisition (equal); investigation (equal); supervision (equal); writing—review and editing (equal).
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the Institutional Review Board of Putuo Hospital affiliated with Shanghai University of Traditional Chinese Medicine (PTEC-A-2016-3-1).
Informed consent
All the participants signed an informed consent before participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Shen, D., Zuo, Q. et al. Efficacy and safety of Huachansu combined with adjuvant chemotherapy in resected colorectal cancer patients: a prospective, open-label, randomized phase II study. Med Oncol 40, 358 (2023). https://doi.org/10.1007/s12032-023-02217-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02217-0