Abstract
Cervical cancer ranks as the fourth most common form of cancer worldwide. There is a large number of situations that may be examined in the developing world. The risk of contracting HPV (Human Papillomavirus) due to poor sanitation and sexual activity is mostly to blame for the disease's alarming rate of expansion. Immunotherapy is widely regarded as one of the most effective medicines available. The immunotherapy used to treat cervical cancer cells relies on inhibitors that block the immune checkpoint. The poly adenosine diphosphate ribose polymer inhibited cervical cancer cells by activating both the programmed death 1 (PD-1) and programmed death ligand 1 (CTLA-1) checkpoints, a strategy that has been shown to have impressive effects. Yet, immunotherapy directed towards tumors that have already been invaded by lymphocytes leaves a positive imprint on the healing process. Immunotherapy is used in conjunction with other treatments, including chemotherapy and radiation, to provide faster and more effective outcomes. In this combination therapy, several medications such as Pembrolizumab, Durvalumab, Atezolizumab, and so on are employed in clinical trials. Recent developments and future predictions suggest that vaccinations will soon be developed with the dual goal of reducing the patient's susceptibility to illness while simultaneously strengthening their immune system. Many clinical and preclinical studies are now investigating the effectiveness of immunotherapy in slowing the progression of cervical cancer. The field of immunotherapy is expected to witness more progress toward improving outcomes.
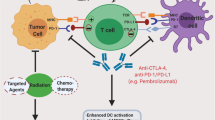
Graphical abstract
Immunotherapies landscape and associated inhibitors for the treatment of Cervical Cancer.





Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of senstivity and are available from the corresponding author upon reasonable request.
Abbreviations
- CAR:
-
Chimeric antigen receptor
- CFU:
-
Colony forming units
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated antigen 4
- DC:
-
Dendritic cell
- FIGO:
-
International Federation of Gynaecology and Obstetrics
- HADC:
-
Histone deacetylases
- HPV:
-
Human papillomavirus
- ICI:
-
Immune checkpoint inhibitor
- MHC:
-
Major histocompatibility complex
- PARP:
-
Poly adenosine diphosphate ribose polymerase
- PD-1:
-
Programmed cell death protein 1
- TCR:
-
T cell receptor
- TIL:
-
Tumor infiltrate lymphocytes
References
American Cancer Society. Key statistics for cervical cancer. American Cancer Society. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics. Accessed 21 Dec 2019.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–61. https://doi.org/10.1056/NEJM199904153401503.
Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137–43. https://doi.org/10.1056/NEJM199904153401501.
Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–53. https://doi.org/10.1056/NEJM199904153401502.
Stehman FB, Ali S, Keys HM, Muderspach LI, Chafe WE, Gallup DG, Walker JL, Gersell D. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197(5):503.e1. https://doi.org/10.1016/j.ajog.2007.08.003.
Vale CL, Tierney JF, Davidson SE, Drinkwater KJ, Symonds P. Substantial improvement in UK cervical cancer survival with chemoradiotherapy: results of a Royal College of Radiologists’ audit. Clin Oncol. 2010;22(7):590–601. https://doi.org/10.1016/j.clon.2010.06.002.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Long HJ III, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, Miller DS, Eaton LA, Fiorica JV. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23(21):4626–33. https://doi.org/10.1200/JCO.2005.10.021.
Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649. https://doi.org/10.1200/JCO.2009.21.8909.
Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25(20):2952–65. https://doi.org/10.1200/JCO.2007.10.8324.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. https://doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F.
Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25:2–3. https://doi.org/10.1002/rmv.1822.
Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(1):S12–7. https://doi.org/10.1016/j.ygyno.2010.04.004.
Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol. 2019;9:682. https://doi.org/10.3389/fonc.2019.00682.
Naumann RW, Leath CA III. Advances in immunotherapy for cervical cancer. Curr Opin Oncol. 2020;32(5):481. https://doi.org/10.1097/CCO.0000000000000663.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Cervical cancer screening guidelines. Cervical Cancer Screening Guidelines Cancer Council (cited 5 May 2023). https://www.cancer.org.au/clinical-guidelines/cervical-cancer/cervical-cancer-screening.
Fu YS, Berek JS. Minimal cervical cancer: definition and histology. In: Grundmann E, Beck L, editors. Minimal neoplasia: diagnosis and therapy. Recent results in cancer research. Berlin: Springer; 1988. p. 47–56.
Moore DH. Cervical cancer. Obstet Gynecol. 2006;107(5):1152–61.
Creasman WT. New gynecologic cancer staging. Gynecol Oncol. 1995;58:157–8.
Bosch FX, Muñoz N. The viral etiology of cervical cancer. Virus Res. 2002;89(2):183–90.
Jones DL, Münger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71(4):2905–12.
Jones EE, Wells SI. Cervical cancer and human papillomaviruses: inactivation of retino-blastoma and other tumor suppressor pathways. Curr Mol Med. 2006;6(7):795–808.
Helt AM, Funk JO, Galloway DA. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. J Virol. 2002;76(20):10559–68.
Wentzensen N, Vinokurova S, von Knebel DM. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004;64(11):3878–84.
Ogilvie G, Nakisige C, Huh WK, Mehrotra R, Franco EL, Jeronimo J. Optimizing secondary prevention of cervical cancer: recent advances and future challenges. Int J Gynecol Obstet. 2017;138:15–9.
Su TH, Chang TY, Lee YJ, Chen CK, Liu HF, Chu CC, Lin M, Wang PT, Huang WC, Chen TC, Yang YC. CTLA-4 gene and susceptibility to human papillomavirus-16-associated cervical squamous cell carcinoma in Taiwanese women. Carcinogenesis. 2007;28(6):1237–40. https://doi.org/10.1093/carcin/bgm043.
Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, Van Der Burg SH. Tumor-expressed B7–H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–7. https://doi.org/10.1158/1078-0432.CCR-09-1652.
Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53. https://doi.org/10.1158/1078-0432.CCR-04-1469.
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML. Tumor B7–H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. https://doi.org/10.1158/0008-5472.CAN-05-4303.
Ghebeh H, Mohammed S, Al-Omair A, Qattant A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Amer SB, Tulbah A, Ajarim D. The B7–H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–8. https://doi.org/10.1593/neo.05733.
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta histochem. 2006;108(1):19–24.
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–5. https://doi.org/10.1073/pnas.0611533104.
Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase 2 keynote-158 study. Early Phase Clin Stud Nov Immunother Oncol. 2019;37:117.
Chen R, Yang W, Li Y, Cheng X, Nie Y, Liu D, Wang H. Effect of immunotherapy on the immune microenvironment in advanced recurrent cervical cancer. Int Immunopharmacol. 2022;106: 108630. https://doi.org/10.1016/j.intimp.2022.108630.
Hassumi-Fukasawa MK, Miranda-Camargo FA, Zanetti BR, Galano DF, Ribeiro-Silva A, Soares EG. Expression of BAG-1 and PARP-1 in precursor lesions and invasive cervical cancer associated with human papillomavirus (HPV). Pathol Oncol Res. 2012;18:929–37. https://doi.org/10.1007/s12253-012-9523-y.
Mauricio D, Zeybek B, Tymon-Rosario J, Harold J, Santin AD. Immunotherapy in cervical cancer. Curr Oncol Rep. 2021;23:1–2. https://doi.org/10.1007/s11912-021-01052-8.
Verma J, Monk BJ, Wolfson AH. New strategies for multimodality therapy in treating locally advanced cervix cancer. Semin Radiat Oncol. 2016;26(4):344–8. https://doi.org/10.1016/j.semradonc.2016.05.003.
Naumann RW, Oaknin A, Meyer T, Lopez-Picazo JM, Lao C, Bang YJ, Boni V, Sharfman WH, Park JC, Devriese LA, Harano K. Efficacy and safety of nivolumab (Nivo)+ ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol. 2019;30:v898–9. https://doi.org/10.1093/annonc/mdz394.059.
Mortezaee K. Immune escape: a critical hallmark in solid tumors. Life Sci. 2020;258: 118110. https://doi.org/10.1016/j.lfs.2020.118110.
Callahan MK, Flaherty CR, Postow MA. Checkpoint blockade for the treatment of advanced melanoma. In: Melanoma. Cham: Springer, 2016, pp. 231–50. https://doi.org/10.1007/978-3-319-22539-5_9.
Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial–mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition ERK inhibition prevents EMT in lung cancer cells. Cancer Res. 2014;74(1):309–19. https://doi.org/10.1158/0008-5472.CAN-12-4721.
Ferrall L, Lin KY, Roden RB, Hung CF, Wu TC. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res. 2021;27(18):4953–73. https://doi.org/10.1158/1078-0432.CCR-20-2833.
Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4+ FOXP3+ regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8(1):59–66. https://doi.org/10.1038/cmi.2010.56.
Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, Restifo NP. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543. https://doi.org/10.1200/JCO.2014.58.9093.
Stevanović S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong ML, Somerville RP, Klebanoff CA, Kammula US, Sherry RM, Yang JC. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–93. https://doi.org/10.1158/1078-0432.CCR-18-2722.
Hinrichs CS, Doran SL, Stevanovic S, Adhikary S, Mojadidi M, Kwong ML, Faquin WC, Feldman S, Somerville R, Sherry RM, Yang JC. A phase I/II clinical trial of E6 T-cell receptor gene therapy for human papillomavirus (HPV)-associated epithelial cancers. https://doi.org/10.1200/JCO.2017.35.15_suppl.3009.
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21. https://doi.org/10.1038/nature22991.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. https://doi.org/10.1126/science.aaa4971.
Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 1993;53(11):2597–602.
Heaton KM, Ju G, Grimm EA. Induction of lymphokine-activated killing with reduced secretion of interleukin-1β, tumor necrosis factor-α, and interferon-γ by interleukin-2 analogs. Ann Surg Oncol. 1994;1:198–203. https://doi.org/10.1007/BF02303524.
Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Investig. 2014;124(1):99–110. https://doi.org/10.1172/JCI46266.
Zhang L, Zhao Y, Tu Q, Xue X, Zhu X, Zhao KN. The roles of programmed cell death ligand-1/programmed cell death-1 (PD-L1/PD-1) in HPV-induced cervical cancer and potential for their use in blockade therapy. Curr Med Chem. 2021;28(5):893–909. https://doi.org/10.2174/0929867327666200128105459.
Yu L, Lanqing G, Huang Z, Xin X, Minglin L, Fa-Hui L, Zou H, Min J. T cell immunotherapy for cervical cancer: challenges and opportunities. Front Immunol. 2023;14:1105265. https://doi.org/10.3389/fimmu.2023.1105265.
Senkomago V, Henley SJ, Thomas CC, et al. Human papillomavirus-attributable cancers—United States, 2012–2016. Morbid Mortal Wkly Rep. 2019;68(33):724–8.
Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–46.
Thompson-Harvey A, Yetukuri M, Hansen AR, et al. Rising incidence of late-stage head and neck cancer in the United States. Cancer. 2020;126(5):1090–101.
Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, Hung CF. Current state in the development of candidate therapeutic HPV vaccines. Exp Rev Vaccines. 2016;15(8):989–1007. https://doi.org/10.1586/14760584.2016.1157477.
Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26(41):5315–20. https://doi.org/10.1016/j.vaccine.2008.07.036.
Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, Alcocer-Gonzalez JM, Tamez-Guerra RS, de Oca-Luna RM, Langella P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53(5):427–33. https://doi.org/10.1099/jmm.0.05472-0.
Cortes-Perez NG, Azevedo V, Alcocer-González JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, Gruss A, Langella P, Bermúdez-Humarán LG. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J Drug Target. 2005;13(2):89–98. https://doi.org/10.1080/10611860400024219.
Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, Yasugi T. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28(16):2810–7. https://doi.org/10.1016/j.vaccine.2010.02.005.
Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9(10):1176–87. https://doi.org/10.1016/j.micinf.2007.05.005.
Chen Z, Ozbun L, Chong N, Wallecha A, Berzofsky JA, Khleif SN. Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+ FoxP3− and CD8+ T cells LLO enhances vaccine efficacy by expanding non-Tregs. Cancer Immunol Res. 2014;2(9):911–22. https://doi.org/10.1158/2326-6066.Cir-13-0197.
Casarin J, Buda A, Bogani G, Fanfani F, Papadia A, Ceccaroni M, Malzoni M, Pellegrino A, Ferrari F, Greggi S, Uccella S. Predictors of recurrence following laparoscopic radical hysterectomy for early-stage cervical cancer: a multi-institutional study. Gynecol Oncol. 2020;159(1):164–70. https://doi.org/10.1016/j.ygyno.2020.06.493.
Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, Hung CF. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15(8):989–1007. https://doi.org/10.1586/14760584.2016.1157477.
Lee SJ, Yang A, Wu TC, Hung CF. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016. https://doi.org/10.3802/jgo.2016.27.e51.
Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–77. https://doi.org/10.1056/NEJMoa0810097.
Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–47. https://doi.org/10.1056/NEJMoa0810097.
van Poelgeest MI, Welters MJ, Vermeij R, Stynenbosch LF, Loof NM, Berends-van der Meer DM, Löwik MJ, Hamming IL, van Esch EM, Hellebrekers BW, van Beurden M. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin Cancer Res. 2016;22(10):2342–50. https://doi.org/10.1158/1078-0432.CCR-15-2594.
Lee SJ, Yang A, Wu TC, Hung CF. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016. https://doi.org/10.3802/jgo.2016.27.e51.
Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, Pai SI, Chen PJ, Lin CT, Boyd DA, Wu TC. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11(12):1011–8. https://doi.org/10.1038/sj.gt.3302252.
Choi YJ, Hur SY, Kim TJ, Hong SR, Lee JK, Cho CH, Park KS, Woo JW, Sung YC, Suh YS, Park JS. A Phase II, prospective, randomized, multicenter, open-label study of GX-188E, an HPV DNA vaccine, in patients with cervical intraepithelial neoplasia 3A Phase II study of a therapeutic HPV DNA vaccine in CIN 3. Clin Cancer Res. 2020;26(7):1616–23. https://doi.org/10.1158/1078-0432.CCR-19-1513.
Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149(1):1–6. https://doi.org/10.1084/jem.149.1.1.
Boer MC, Joosten SA, Ottenhoff TH. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front Immunol. 2015;6:217. https://doi.org/10.3389/fimmu.2015.00217.
Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010;40(7):1916–26. https://doi.org/10.1002/eji.201040310.
Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des. 2005;11(27):3485–500. https://doi.org/10.2174/138161205774414565.
Kim JH, Kang TH, Noh KH, Bae HC, Kim SH, Do Yoo Y, Seong SY, Kim TW. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8+ T cell-mediated cell death. Immunol Lett. 2009;122(1):58–67. https://doi.org/10.1016/j.imlet.2008.12.006.
Wang TL, Ling M, Shih IM, Pham T, Pai SI, Lu Z, Kurman RJ, Pardoll DM, Wu TC. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene Ther. 2000;7(9):726–33. https://doi.org/10.1038/sj.gt.3301160.
Tsuda N, Watari H, Ushijima K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin J Cancer Res. 2016;28(2):241. https://doi.org/10.21147/j.issn.1000-9604.2016.02.14.
Ratnasabapathy Y, Chi-Lun Lee A, Feigin V, Anderson C. Blood pressure lowering interventions for preventing dementia in patients with cerebrovascular disease (Protocol). Cochrane Database Syst Rev. 2003. https://doi.org/10.1002/14651858.CD004034.pub3.
Brucker SY, Ulrich UA. Surgical treatment of early-stage cervical cancer. Oncol Res Treat. 2016;39(9):508–14. https://doi.org/10.1002/14651858.CD005342.pub4.
Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58(3):317–24. https://doi.org/10.1007/s00262-008-0576-4.
Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, Monie A. Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–92. https://doi.org/10.1158/1078-0432.CCR-08-0037.
Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, van Veelen PA, Melief CJ, van der Burg SH, Arens R. Tumor eradication by cisplatin is sustained by CD80/86-mediated costimulation of CD8+ T cells. Cancer Res. 2016;76(20):6017–29. https://doi.org/10.1158/0008-5472.CAN-16-0881.
Lee SY, Kang TH, Knoff J, Huang Z, Soong RS, Alvarez RD, Hung CF, Wu TC. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer Immunol Immunother. 2013;62:1175–85. https://doi.org/10.1007/s00262-013-1421-y.
Melief CJ, Welters MJ, Vergote I, Kroep JR, Kenter GG, Ottevanger PB, Tjalma WA, Denys H, van Poelgeest MI, Nijman HW, Reyners AK. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med. 2020;12(535):eaaz8235. https://doi.org/10.1126/scitranslmed.aaz8235.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. https://doi.org/10.1084/jem.20052494.
Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE. 2012;7(3): e32542. https://doi.org/10.1371/journal.pone.0032542.
Kuwabara M, Takahashi K, Inanami O. Induction of apoptosis through the activation of SAPK/JNK followed by the expression of death receptor Fas in X-irradiated cells. J Radiat Res. 2003;44(3):203–9. https://doi.org/10.1269/jrr.44.203.
Immunotherapy A. Irradiation of tumor cells up-regulates Fas. J Immunol. 2003;170:6338–47. https://doi.org/10.4049/jimmunol.170.12.6338.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9. https://doi.org/10.1038/nm1622.
Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. https://doi.org/10.4049/jimmunol.174.12.7516.
Dustin ML, Demaria S, Babb JS, Schneider RJ, Formenti SC, Braunstein S, Badura M, Cameron TO, Matsumura S, Wang B, Kawashima N. Radiation-induced CXCL16 release. J Immunol. 2008;181:3099–107. https://doi.org/10.4049/jimmunol.181.5.3099.
Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2):728–34. https://doi.org/10.1158/1078-0432.728.11.2.
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody fractionated radiation synergizes with immunotherapy. Clin Cancer Res. 2009;15(17):5379–88. https://doi.org/10.1158/1078-0432.CCR-09-0265.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124(2):687–95. https://doi.org/10.1172/JCI67313.
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68. https://doi.org/10.1158/0008-5472.CAN-14-1258.
Lyu M, Shen Y, Beharee N, Lu J, Deng F, Wang J. The combined use of chemotherapy and radiotherapy with PD-1 inhibitor, pembrolizumab, in advanced cervical cancer: a case report. OncoTargets Ther. 2020;13:4465. https://doi.org/10.2147/OTT.S245190.
Feng CH, Mell LK, Sharabi AB, McHale M, Mayadev JS. Immunotherapy with radiotherapy and chemoradiotherapy for cervical cancer. Semin Radiat Oncol. 2020;30(4):273–80. https://doi.org/10.1016/j.semradonc.2020.05.003.
Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, Petit R. A randomized phase 2 study of ADXS11-001 Listeria monocytogenes–listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018. https://doi.org/10.1097/IGC.0000000000001235.
Study of ADXS11-001 in subjects with high risk locally advanced cervical cancer—full text view. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02853604.
Frenel JS, Le Tourneau C, O'Neil BH, Ott PA, Piha-Paul SA, Gomez-Roca CA, Brummelen EV, Rugo HS, Thomas S, Saraf S, Chen M. Pembrolizumab in patients with advanced cervical squamous cell cancer: preliminary results from the phase Ib KEYNOTE-028 study. https://doi.org/10.1200/JCO.2016.34.15_suppl.5515.
Chung HC, Schellens JH, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Wang J, Zeigenfuss S, Pruitt SK. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase 2 KEYNOTE-158 study.
Efficacy and safety study of first-line treatment with pembrolizumab (MK-3475) plus chemotherapy versus placebo plus chemotherapy in women with persistent, recurrent, or metastatic cervical cancer (MK-3475-826/Keynote-826)—View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03635567.
An Investigational Immuno-therapy Study to Investigate the Safety and Effectiveness of Nivolumab, and Nivolumab Combination Therapy in Virus-Associated Tumors—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02488759.
SHR-1210 in combination with apatinib in patients with metastatic, persistent, or recurrent cervical cancer. Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03816553.
Image guided IMRT, radio chemotherapy and MRI-based IGABT in locally advanced cervical cancer. Image Guided IMRT, Radiochemotherapy and MRI-Based IGABT in Locally Advanced Cervical Cancer—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03617133.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22–30. https://doi.org/10.3802/jgo.2016.27.e43.
Li B, Cui Y, Nambiar DK, Sunwoo JB, Li R. The immune subtypes and landscape of squamous cell carcinoma immune landscape of squamous cell carcinoma. Clin Cancer Res. 2019;25(12):3528–37. https://doi.org/10.1158/1078-0432.Ccr-18-4085.
Burmeister CA, Khan SF, Schäfer G, Mbatani N, Adams T, Moodley J, Prince S. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 2022;2022:200238. https://doi.org/10.1016/j.tvr.2022.200238.
Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016. https://doi.org/10.3802/jgo.2016.27.e43.
Hamid NA, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci. 2009;66:1700–17. https://doi.org/10.1007/s00018-009-8631-7.
Acknowledgements
All the authors are thankful to library of Galgotias University for providing the material and support to us.
Funding
There is no financial support is required for this review article. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
We affirm that all the authors have seen, prepared, and agreed to the submission of the paper and their inclusion of name(s) as co-author(s).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, A., Yadav, S. & Alam, M.A. Immunotherapies landscape and associated inhibitors for the treatment of cervical cancer. Med Oncol 40, 328 (2023). https://doi.org/10.1007/s12032-023-02188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02188-2