Abstract
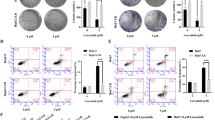
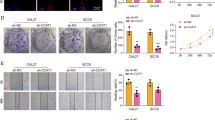
EZH2, a highly conserved histone methyltransferase, plays an essential role in tumorigenesis and development. The inhibitor of EZH2 tazemetostat has been approved to treat metastatic or locally advanced epithelioid sarcoma and recurrent or refractory follicular lymphoma. However, the effect of tazemetostat alone or in combination with other drugs in esophageal cancer has not been reported. In this study, we found that EZH2 was highly expressed in esophageal cancer at both mRNA and protein levels through transcriptomic and proteomic analyses. Furthermore, the results of CCK8, colony formation, cell cycle and cell apoptosis assays revealed that tazemetostat exerted an antitumour effect on esophageal cancer cells. Mechanistically, RNA-sequencing analysis of the tazemetostat-treated cells and vehicle-treated ones suggested that tazemetostat mainly inhibited the c-Myc signaling pathway and its targets, which was validated by western blotting. JQ-1, an inhibitor of bromodomain 4, was proven to attenuate c-Myc signaling in tumors. Thus, a therapeutic strategy based on tazemetostat in combination with JQ-1 is promising. The results demonstrated that tazemetostat and JQ-1 had a synergistic effect in vitro and in vivo for esophageal cancer.






Similar content being viewed by others
Data Availability
All the data supporting the findings of this study are available upon reasonable request. Raw data for RNA-seq were deposited in the SRA database under accession number SUB12215728.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–9.
Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647(1–2):21–9.
He A, Shen X, Ma Q, et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012;26(1):37–42.
Lee JM, Lee JS, Kim H, et al. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol Cell. 2012;48(4):572–86.
Gunawan M, Venkatesan N, Loh JT, et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat Immunol. 2015;16(5):505–16.
Kim E, Kim M, Woo DH, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839–52.
Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science. 2012;338(6113):1465–9.
Kim J, Lee Y, Lu X, et al. Polycomb- and methylation-independent roles of EZH2 as a transcription activator. Cell Rep. 2018;25(10):2808–2820e2804.
Nutt SL, Keenan C, Chopin M, et al. EZH2 function in immune cell development. Biol Chem. 2020;401(8):933–43.
Ito T, Teo YV, Evans SA, et al. Regulation of Cellular Senescence by polycomb chromatin modifiers through distinct DNA damage- and histone methylation-dependent pathways. Cell Rep. 2018;22(13):3480–92.
Batool A, Jin C, Liu YX. Role of EZH2 in cell lineage determination and relative signaling pathways. Front bioscience (Landmark edition). 2019;24:947–60.
Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–9.
Collett K, Eide GE, Arnes J, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12(4):1168–74.
Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–73.
Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11606–11.
Liu F, Gu L, Cao Y et al. Aberrant overexpression of EZH2 and H3K27me3 serves as poor prognostic biomarker for esophageal squamous cell carcinoma patients. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 2016, 21(1):80–90.
First EZH2. Inhibitor approved-for Rare Sarcoma. Cancer Discov. 2020;10(3):333–4.
Hoy SM, Tazemetostat. First Approval Drugs. 2020;80(5):513–21.
Duffy MJ, O’Grady S, Tang M, et al. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94:102154.
Bhadury J, Nilsson LM, Veppil Muralidharan S, et al. BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in myc-induced murine lymphoma. Proc Natl Acad Sci U S A. 2014;111(26):E2721–2730.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81.
Ding N, You A, Tian W, et al. Chidamide increases the sensitivity of non-small cell Lung Cancer to Crizotinib by decreasing c-MET mRNA methylation. Int J Biol Sci. 2020;16(14):2595–611.
Huang Z, Zhou W, Li Y, et al. Novel hybrid molecule overcomes the limited response of solid tumours to HDAC inhibitors via suppressing JAK1-STAT3-BCL2 signaling. Theranostics. 2018;8(18):4995–5011.
Xue ST, Zheng B, Cao SQ, et al. Long non-coding RNA LINC00680 functions as a ceRNA to promote esophageal squamous cell carcinoma progression through the miR-423-5p/PAK6 axis. Mol Cancer. 2022;21(1):69.
He LR, Liu MZ, Li BK, et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer. 2010;127(1):138–47.
Wang HR, Wang MH, Lian GY, et al. [Expression of enhancer of zeste homolog 2 in esophageal squamous cell carcinoma and its prognostic value in postoperative patients]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2015;35(1):99–102.
Yamada A, Fujii S, Daiko H, et al. Aberrant expression of EZH2 is associated with a poor outcome and P53 alteration in squamous cell carcinoma of the esophagus. Int J Oncol. 2011;38(2):345–53.
Sun J, Cai X, Yung MM, et al. miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer. Oncogene. 2019;38(4):564–80.
Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112(10):4202–12.
Neri F, Zippo A, Krepelova A, et al. Myc regulates the transcription of the PRC2 gene to control the expression of developmental genes in embryonic stem cells. Mol Cell Biol. 2012;32(4):840–51.
Barbieri I, Cannizzaro E, Dawson MA. Bromodomains as therapeutic targets in cancer. Brief Funct Genomics. 2013;12(3):219–30.
Funding
This work was supported by start-up funds for the introduction of talents from the First Affiliated Hospital of Xiamen University (3502Z20214ZD3010), Health Youth Scientific Research Project of Fujian Province (2020QNB057; 2021QNB015), and the Natural Science Foundation of Fujian Province, China (2020J011238; 2021J05291; 2022J05304).
Author information
Authors and Affiliations
Contributions
Feng Ye and Nan Ding generated the ideas for the study and wrote the manuscript. Nan Ding and Abin You conducted the experiments of the clinical specimens and performed the molecular biological experiments with the assistance of Senxia Zhao. Hu Yang and Chunping Lai did the animal experiment. All authors participated in editing the paper. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Xiamen University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, N., You, A., Zhao, S. et al. EZH2 inhibitor Tazemetostat synergizes with JQ-1 in esophageal cancer by inhibiting c-Myc signaling pathway. Med Oncol 40, 281 (2023). https://doi.org/10.1007/s12032-023-02147-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02147-x