Abstract
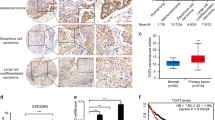
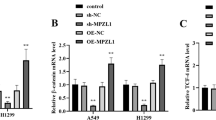
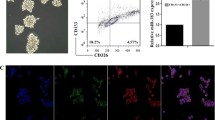
Lung cancer is the leading cause of malignant tumor-related deaths worldwide. The presence of tumor-initiating cells in lung cancer leads to tumor recurrence, metastasis, and resistance to conventional treatment. Cleavage and polyadenylation specificity factor 4 (CPSF4) activation in tumor cells contributes to the poor prognosis of lung cancer. However, the precise biological functions and molecular mechanisms of CPSF4 in the regulation of tumor-initiating cells remain unclear. We demonstrated that CPSF4 promotes tumor-initiating phenotype and confers chemoresistance to paclitaxel both in vitro and in vivo. Mechanistically, we showed that CPSF4 binds to the promoters of vascular endothelial growth factor (VEGF) and neuropilin-2 (NRP2) and activated their transcription. In addition, we showed that CPSF4/VEGF/NRP2-mediated tumor-initiating phenotype and chemoresistance through TAZ induction. Furthermore, analysis of clinical data revealed that lung cancer patients with high CPSF4 expression exhibit high expression levels of VEGF, NRP2, and TAZ and that expression of these proteins are positively correlated with poor prognosis. Importantly, selective inhibition of VEGF, NRP2, or TAZ markedly suppressed CPSF4-mediated tumor-initiating phenotype and chemoresistance. Our findings reveal the mechanism of CPSF4 modulating tumor-initiating phenotype and chemoresistance in lung cancer and indicate that the CPSF4-VEGF-NRP2-TAZ signaling pathway may be a prognosis marker and therapeutic target in lung cancer.






Similar content being viewed by others
Data availability
The datasets are available in the National Center of Biotechnology Information (NCBI) GEO (https://www.ncbi.nlm.nih.gov/geo/). Datasets GSE41271 and GSE37745 were used in the present study.
Abbreviations
- CPSF4:
-
Cleavage and polyadenylation specificity factor 4
- ChIP:
-
Chromatin immunoprecipitation
- VEGF:
-
Vascular endothelial growth factor
- NRP:
-
Neuropilin
- TICs:
-
Tumor-initiating cells
- PTX:
-
Paclitaxel
- GEO:
-
Gene Expression Omnibus
- GSEA:
-
Gene set enrichment analysis
- PVDF:
-
Polyvinylidene difluoride
- IHC:
-
Immunohistochemical
- SD:
-
Standard deviation
- COUP-TFII:
-
COUP transcription factor II
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Jurisic V, Vukovic V, Obradovic J, Gulyaeva LF, Kushlinskii NE, Djordjevic N. EGFR polymorphism and survival of NSCLC patients treated with TKIs: a systematic review and meta-analysis. J Oncol. 2020;2020:1973241.
The L. Lung cancer: some progress, but still a lot more to do. Lancet. 2019;394:1880.
Prabavathy D, Swarnalatha Y, Ramadoss N. Lung cancer stem cells-origin, characteristics and therapy. Stem Cell Investig. 2018;5:6.
Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019;8:840.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Yang Q, Fan W, Zheng Z, Lin S, Liu C, Wang R, Li W, Zuo Y, Sun Y, Hu S, et al. Cleavage and polyadenylation specific factor 4 promotes colon cancer progression by transcriptionally activating hTERT. Biochim Biophys Acta Mol Cell Res. 2019;1866:1533–43.
Wu J, Miao J, Ding Y, Zhang Y, Huang X, Zhou X, Tang R. MiR-4458 inhibits breast cancer cell growth, migration, and invasiveness by targeting CPSF4. Biochem Cell Biol. 2019;97:722–30.
Lee K, Zheng Q, Lu Q, Xu F, Qin G, Zhai Q, Hong R, Chen M, Deng W, Wang S. CPSF4 promotes triple negative breast cancer metastasis by upregulating MDM4. Signal Transduct Target Ther. 2021;6:184.
Chen W, Guo W, Li M, Shi D, Tian Y, Li Z, Wang J, Fu L, Xiao X, Liu QQ, et al. Upregulation of cleavage and polyadenylation specific factor 4 in lung adenocarcinoma and its critical role for cancer cell survival and proliferation. PLoS One. 2013;8:e82728.
Chen W, Qin L, Wang S, Li M, Shi D, Tian Y, Wang J, Fu L, Li Z, Guo W, et al. CPSF4 activates telomerase reverse transcriptase and predicts poor prognosis in human lung adenocarcinomas. Mol Oncol. 2014;8:704–16.
Li Z, Xu X, Li Y, Zou K, Zhang Z, Liao Y, Zhao X, Jiang W, Yu W, Guo W, et al. Synergistic antitumor effect of BKM120 with prima-1met via inhibiting PI3K/AKT/mTOR and CPSF4/hTERT signaling and reactivating mutant P53. Cell Physiol Biochem. 2018;45:1772–86.
Zhang N, Xie Y, Tai Y, Gao Y, Guo W, Yu W, Li J, Feng X, Hao J, Zhao X, et al. Bufalin inhibits hTERT expression and colorectal cancer cell growth by targeting CPSF4. Cell Physiol Biochem. 2016;40:1559–69.
Wang X, Dong J, Li X, Cheng Z, Zhu Q. CPSF4 regulates circRNA formation and microRNA mediated gene silencing in hepatocellular carcinoma. Oncogene. 2021;40:4338–51.
Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14.
Verseijden F, Jahr H, Posthumus-van Sluijs SJ, Ten Hagen TL, Hovius SE, Seynhaeve AL, van Neck JW, van Osch GJ, Hofer SO. Angiogenic capacity of human adipose-derived stromal cells during adipogenic differentiation: an in vitro study. Tissue Eng Part A. 2009;15:445–52.
Kane NM, Xiao Q, Baker AH, Luo Z, Xu Q, Emanueli C. Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation). Pharmacol Ther. 2011;129:29–49.
Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25:831–44.
Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–8.
Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403.
Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J Jr, Fischer W, Lukas J, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–20.
Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–25.
Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–26.
Neufeld G, Kessler O, Herzog Y. The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90.
Elaimy AL, Guru S, Chang C, Ou J, Amante JJ, Zhu LJ, Goel HL, Mercurio AM. VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Sci Signal. 2018;11:eaao6897.
Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL, et al. GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation EMBO. Mol Med. 2013;5:488–508.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50.
Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M. TNF-alpha induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr Biol. 2011;239:115–22.
Vuletic A, Konjevic G, Milanovic D, Ruzdijic S, Jurisic V. Antiproliferative effect of 13-cis-retinoic acid is associated with granulocyte differentiation and decrease in cyclin B1 and Bcl-2 protein levels in G0/G1 arrested HL-60 cells. Pathol Oncol Res. 2010;16:393–401.
Zheng F, Yue C, Li G, He B, Cheng W, Wang X, Yan M, Long Z, Qiu W, Yuan Z, et al. Nuclear AURKA acquires kinase-independent transactivating function to enhance breast cancer stem cell phenotype. Nat Commun. 2016;7:10180.
Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–38.
Alguacil-Nunez C, Ferrer-Ortiz I, Garcia-Verdu E, Lopez-Pirez P, Llorente-Cortijo IM, Sainz B Jr. Current perspectives on the crosstalk between lung cancer stem cells and cancer-associated fibroblasts. Crit Rev Oncol Hematol. 2018;125:102–10.
Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82.
Niland S, Eble JA. Neuropilins in the context of tumor vasculature. Int J Mol Sci. 2019;20:639.
Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci. 2019;20:490.
Napolitano V, Tamagnone L. Neuropilins controlling cancer therapy responsiveness. Int J Mol Sci. 2019;20:2049.
Tang Z, Yu W, Zhang C, Zhao S, Yu Z, Xiao X, Tang R, Xuan Y, Yang W, Hao J, et al. CREB-binding protein regulates lung cancer growth by targeting MAPK and CPSF4 signaling pathway. Mol Oncol. 2016;10:317–29.
Yi C, Wang Y, Zhang C, Xuan Y, Zhao S, Liu T, Li W, Liao Y, Feng X, Hao J, et al. Cleavage and polyadenylation specific factor 4 targets NF-kappaB/cyclooxygenase-2 signaling to promote lung cancer growth and progression. Cancer Lett. 2016;381:1–13.
Kiefer H, Mizutani A, Iemura S, Natsume T, Ando H, Kuroda Y, Mikoshiba K. Inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) associates with components of the mRNA 3’ processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation. J Biol Chem. 2009;284:10694–705.
Aragaki M, Takahashi K, Akiyama H, Tsuchiya E, Kondo S, Nakamura Y, Daigo Y. Characterization of a cleavage stimulation factor, 3’ pre-RNA, subunit 2, 64 kDa (CSTF2) as a therapeutic target for lung cancer. Clin Cancer Res. 2011;17:5889–900.
Sung TY, Kim M, Kim TY, Kim WG, Park Y, Song DE, Park SY, Kwon H, Choi YM, Jang EK, et al. Negative expression of CPSF2 predicts a poorer clinical outcome in patients with papillary thyroid carcinoma. Thyroid. 2015;25:1020–5.
Bolli N, Payne EM, Rhodes J, Gjini E, Johnston AB, Guo F, Lee JS, Stewart RA, Kanki JP, Chen AT, et al. cpsf1 is required for definitive HSC survival in zebrafish. Blood. 2011;117:3996–4007.
Nilubol N, Boufraqech M, Zhang L, Kebebew E. Loss of CPSF2 expression is associated with increased thyroid cancer cellular invasion and cancer stem cell population, and more aggressive disease. J Clin Endocrinol Metab. 2014;99:E1173-82.
Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, et al. CD10(+)GPR77(+) Cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841-856 e816.
Lum C, Alamgeer M. Technological and therapeutic advances in advanced small cell lung cancer. Cancers (Basel). 2019;11:1570.
Po A, Silvano M, Miele E, Capalbo C, Eramo A, Salvati V, Todaro M, Besharat ZM, Catanzaro G, Cucchi D, et al. Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma. Oncogene. 2017;36:4641–52.
Qin J, Wu SP, Creighton CJ, Dai F, Xie X, Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, et al. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–40.
Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, Schober M. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014;5:4511.
Goel HL, Pursell B, Shultz LD, Greiner DL, Brekken RA, Vander Kooi CW, Mercurio AM. P-Rex1 promotes resistance to VEGF/VEGFR-targeted therapy in prostate cancer. Cell Rep. 2016;14:2193–208.
Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and alpha6/beta4-integrins to increase YAP1/Np63alpha-dependent epidermal cancer stem cell survival. Oncogene. 2018;37:4711–22.
Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–76.
Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, de Assuncao TM, Cao Y, Szabolcs A, Thorgeirsson S, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012;72:4047–59.
Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res. 2010;8:1063–73.
Li H, Takayama K, Wang S, Shiraishi Y, Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I, et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74:1297–305.
Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–94.
Frezzetti D, Gallo M, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, Normanno N, De Luca A. VEGF as a potential target in lung cancer. Expert Opin Ther Targets. 2017;21:959–66.
Manzo A, Montanino A, Carillio G, Costanzo R, Sandomenico C, Normanno N, Piccirillo MC, Daniele G, Perrone F, Rocco G, et al. Angiogenesis inhibitors in NSCLC. Int J Mol Sci. 2017;18:2021.
Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming XuJ, Peng Yong W, Langer B, Delmar P, Scherer SJ, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119–27.
Jurisic V. Multiomic analysis of cytokines in immuno-oncology. Expert Rev Proteomics. 2020;17:663–74.
Lopez de Andres J, Grinan-Lison C, Jimenez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J Hematol Oncol. 2020;13:136.
Aponte PM, Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017;2017:5619472.
Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–14.
Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402.
Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87.
Acknowledgements
We thank the members of FeiMeng Zheng’s, WuGuo Deng’s, and ShuSen Wang’s laboratory for their critical comments and technical support.
Funding
This work was supported by funds from the National Natural Science Foundation of China, China (81401905 to W.B.C.) and the National Natural Science Foundation of Guangdong, China (2017A030313489 to T.Q.).
Author information
Authors and Affiliations
Contributions
The work was done in collaboration with all authors. WBC, FMZ, and GLL contribute to the conception and design of this work and reviewed the manuscript. WBC, YQS, and KS analyzed the raw data. FMZ help analyze data and organize data. YQS and KS designed the experimental methods, completed the experiments, and wrote the manuscript. LLG and LLS provided technical support during completing the experiments. SSW, WGD, and TQ provided critical comments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors agreed to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Sun, K., Gong, L. et al. CPSF4 promotes tumor-initiating phenotype by enhancing VEGF/NRP2/TAZ signaling in lung cancer. Med Oncol 40, 62 (2023). https://doi.org/10.1007/s12032-022-01919-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01919-1