Abstract
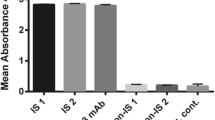
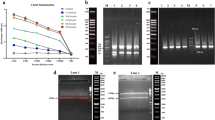
To discover new therapeutic antibodies for treatment of acute myeloid leukemia (AML) without the requirement of a known antigen, a human single-chain variable fragment (scFv) library was used to isolate novel antibody fragments recognizing HL-60 AML cells. After three rounds of biopanning, scFv-expressing phages were selected to test for binding to the target cell by flow cytometry. The clone with highest binding specificity to HL-60 cells (designated y1HL63D6) was further investigated. Fluorescent staining indicated that y1HL63D6 scFv bound to a target located on the cell surface. Whole immunoglobulin, IgG-y1HL63D6 was then generated and tested for the binding against bone marrow mononuclear cells (BMMCs) from AML patients. Significantly higher fluorescent signals were observed for some patient samples when compared to normal BMMCs or non-AML patients’ BMMCs. Next, the IgG-y1HL63D6 format was tested for antibody-dependent cell cytotoxicity (ADCC). The results demonstrated that IgG-y1HL63D6 but not the control antibody, trastuzumab, could mediate specific killing of HL-60 target cells. In conclusion, our results indicate that specific antibodies can be isolated by biopanning whole cells with a non-immunized human scFv antibody phage display library and that the isolated antibody against HL-60 cells showed therapeutic potential.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML:
-
Acute myeloid leukemia
- scFv:
-
Single-chain variable fragment
- IgG:
-
Immunoglobulin G
- VH:
-
Variable heavy
- VL:
-
Variable light
- HC:
-
Heavy chain
- LC:
-
Light chain
- BMMCs:
-
Bone marrow mononuclear cells
- PBMCs:
-
Peripheral blood mononuclear cells
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- MFI:
-
Median fluorescent intensity
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
American cancer society. cancer facts & figures 2021. American Cancer Society. 2021. https://www.cancer.org/research/cancer-facts-statistics. Accessed 8 Mar 2022.
Caron PC, et al. Biological and immunological features of humanized M195 (Anti-CD33) monoclonal antibodies. Cancer Res. 1992;52(24):6761.
Bakker ABH, et al. C-Type Lectin-Like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64(22):8443–50. https://doi.org/10.1158/0008-5472.CAN-04-1659.
Jin L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5(1):31–42. https://doi.org/10.1016/j.stem.2009.04.018.
Krupka, C., et al. Targeting CD157 in AML using a novel, Fc-engineered antibody construct. Oncotarget. 2017;8(22):35707–35717. https://doi.org/10.18632/oncotarget.16060
Kantarjian H, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41. https://doi.org/10.1038/s41408-021-00425-3.
Sidhu SS. Phage display in pharmaceutical biotechnology. Curr Opin Biotechnol. 2000;11(6):610–6. https://doi.org/10.1016/S0958-1669(00)00152-X.
Zhang, Y., et al. Prokaryotic expression of MLAA-34 and generation of a novel human ScFv against MLAA-34 by phage display technology. Oncotarget. 2017;8(24):39077–39086. https://doi.org/10.18632/oncotarget.16590
Crepin R, et al. Whole-cell biopanning with a synthetic phage display library of nanobodies enabled the recovery of follicle-stimulating hormone receptor inhibitors. Biochem Biophys Res Commun. 2017;493(4):1567–72. https://doi.org/10.1016/j.bbrc.2017.10.036.
Yuan QA, et al. Isolation of anti-MISIIR scFv molecules from a phage display library by cell sorter biopanning. Cancer Immunol Immunother. 2008;57(3):367–78. https://doi.org/10.1007/s00262-007-0376-2.
Muraoka S, et al. Effective induction of cell death on adult T-cell leukaemia cells by HLA-DRbeta-specific small antibody fragment isolated from human antibody phage library. J Biochem. 2009;145(6):799–810. https://doi.org/10.1093/jb/mvp039.
Jones ML, et al. Targeting membrane proteins for antibody discovery using phage display. Sci Rep. 2016;6:26240. https://doi.org/10.1038/srep26240.
Drexler HG, et al. Leukemia cell lines: in vitro models for the study of acute promyelocytic leukemia. Leuk Res. 1995;19(10):681–91. https://doi.org/10.1016/0145-2126(95)00036-N.
Corcoran A, et al. Biological evaluation of double point modified analogues of 1,25-Dihydroxyvitamin D(2) as potential anti-leukemic agents. Int J Mol Sci. 2016;17(2):91. https://doi.org/10.3390/ijms17020091.
Li Q, et al. Subcellular localization of DJ-1 in human HL-60 leukemia cells in response to diallyl disulfide treatment. Mol Med Rep. 2016;14(5):4666–72. https://doi.org/10.3892/mmr.2016.5831.
Takahashi, N., et al. Retinoylation (covalent modification by retinoic acid) of Rho-GDIβ in the human myeloid leukemia cell line HL60 and its functional significance. Biochim Biophys Acta. 2016;1861(12, Part A):2011–2019. https://doi.org/10.1016/j.bbalip.2016.10.001
Pansri P, Jaruseranee N, Rangnoi K, Kristensen P, Yamabhai M. A compact phage display human scFv library for selection of antibodies to a wide variety of antigens. BMC Biotechnol. 2009;9(1):6. https://doi.org/10.1186/1472-6750-9-6.
Keller T, et al. Selection of scFv antibody fragments binding to human blood versus lymphatic endothelial surface antigens by direct cell phage display. PLoS ONE. 2015;10(5): e0127169. https://doi.org/10.1371/journal.pone.0127169.
Rangnoi K, et al. Binding characteristic of various antibody formats against aflatoxins. ACS Omega. 2021;6(39):25258–68. https://doi.org/10.1021/acsomega.1c03044.
Bühring HJ, et al. The receptor tyrosine kinase p185HER2 is expressed on a subset of B-lymphoid blasts from patients with acute lymphoblastic leukemia and chronic myelogenous leukemia. Blood. 1995;86(5):1916–23. https://doi.org/10.1182/blood.V86.5.1916.bloodjournal8651916.
van Rhenen A, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11(18):6520–7. https://doi.org/10.1158/1078-0432.CCR-05-0468.
Brian B, Eytan MS. Which are the most promising targets for minimal residual disease-directed therapy in acute myeloid leukemia prior to allogeneic stem cell transplant? Haematologica. 2019;104(8):1521–31. https://doi.org/10.3324/haematol.2018.208587.
Dalton WT Jr, et al. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood. 1988;71(1):242–7. https://doi.org/10.1182/blood.V71.1.242.242.
Mehdipour T, et al. Tailoring subtractive cell biopanning to identify diffuse gastric adenocarcinoma-associated antigens via human scFv antibodies. Immunology. 2020;159(1):96–108. https://doi.org/10.1111/imm.13129.
Hoogenboom HR, et al. Selection-dominant and nonaccessible epitopes on cell-surface receptors revealed by cell-panning with a large phage antibody library. Eur J Biochem. 1999;260(3):774–84. https://doi.org/10.1046/j.1432-1327.1999.00214.x.
Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13(1):37–45. https://doi.org/10.1016/S1074-7613(00)00006-6.
Lagunas-Rangel FA, et al. Acute myeloid leukemia-genetic alterations and their clinical prognosis. Int J Hematol Oncol Stem Cell Res. 2017;11(4):328–39.
Horibata S, et al. Heterogeneity in refractory acute myeloid leukemia. Proc Natl Acad Sci U S A. 2019;116(21):10494. https://doi.org/10.1073/pnas.1902375116.
Collignon A, et al. A chemogenomic approach to identify personalized therapy for patients with relapse or refractory acute myeloid leukemia: results of a prospective feasibility study. Blood Cancer J. 2020;10(6):64. https://doi.org/10.1038/s41408-020-0330-5.
Chanput W, P.V., Wichers H. THP-1 and U937 Cells. In: The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet], C.P. Verhoeckx K, López-Expósito I, et al. Editor. Cham (CH): Springer; 2015. Chapter 14.
Drexler HG, Matsuo Y, MacLeod RAF. Malignant hematopoietic cell lines: in vitro models for the study of erythroleukemia. Leuk Res. 2004;28(12):1243–51. https://doi.org/10.1016/j.leukres.2004.03.022.
Zotova A, Zotov I, Filatov A, Mazurov D. Determining antigen specificity of a monoclonal antibody using genome-scale CRISPR-Cas9 knockout library. J Immunol Methods. 2016;439:8–14. https://doi.org/10.1016/j.jim.2016.09.006.
Tabasinezhad M, Talebkhan Y, Wenzel W, Rahimi H, Omidinia E, Mahboudi F. Trends in therapeutic antibody affinity maturation: from in-vitro towards next-generation sequencing approaches. Immunol Lett. 2019;212:106–13. https://doi.org/10.1016/j.imlet.2019.06.009.
Park S, et al. Quantitative RT-PCR assay of HER2 mRNA expression in formalin-fixed and paraffin-embedded breast cancer tissues. Int J Clin Exp Pathol. 2014;7(10):6752–9.
Rangnoi K, et al. Enhancement and analysis of human antiaflatoxin B1 (AFB1) scFv antibody-ligand interaction using chain shuffling. J Agric Food Chem. 2018;66(22):5713–22. https://doi.org/10.1021/acs.jafc.8b01141.
Sompunga P, et al. Generation of human and rabbit recombinant antibodies for the detection of Zearalenone by phage display antibody technology. Talanta. 2019;201:397–405. https://doi.org/10.1016/j.talanta.2019.04.034.
Khaing, K.K., et al. Application of Recombinant Human scFv Antibody as a Powerful Tool to Monitor Nitrogen Fixing Biofertilizer in Rice and Legume. Microbiol Spectr. 2021;9(3):e0209421. https://doi.org/10.1128/Spectrum.02094-21
Zahavi D, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1(1):7–12. https://doi.org/10.1093/abt/tby002.
Hassenrück F, et al. Sensitive detection of the natural killer cell-mediated cytotoxicity of anti-CD20 antibodies and its impairment by B-Cell receptor pathway inhibitors. Biomed Res Int. 2018;2018:1023490. https://doi.org/10.1155/2018/1023490.
Wang W, et al. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368–368. https://doi.org/10.3389/fimmu.2015.00368.
Fujiwara H, et al. Tissue-restricted T cell alloresponses across HLA barriers: selection and identification of leukemia-restricted CTL in HLA-mismatched stimulator–responder pairs. Bone Marrow Transpl. 2003;32(4):371–8. https://doi.org/10.1038/sj.bmt.1704142.
Horowitz, A., et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145. https://doi.org/10.1126/scitranslmed.3006702
Romain G, et al. Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood. 2014;124(22):3241–9. https://doi.org/10.1182/blood-2014-04-569061.
Vasu S, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127(23):2879–89. https://doi.org/10.1182/blood-2015-11-680546.
Acknowledgements
The authors would like to thank Miss Yuwadee Kitprasong, medical technologist at Sanphasitthiprasong hospital, for patient sample collection. We would also like to thank to all participants who volunteered for this study. We are in dept. of Prof. Dr. Pa-thai Yenchitsomanus from Siriraj Center of Research Excellence for Cancer Immunotherapy, Mahidol University, Thailand for several cell lines and grateful to MY lab members and KAC lab members for excellent technical assistances and advice.
Funding
This research was supported by Thailand Science Research and Innovation (TSRI) (TRF Senior Research grant number RTA6180012) and BIOTEC, the National Science and Technology Development Agency (NSTDA) (grant number P-18–50127), and Ministry of Higher Education, Science, Research and Innovation (MHESI) (grant number 256101A3040017). TS was co-supported by the Royal Golden Jubilee PhD program of Thailand (rgj.trf.or.th) and Suranaree University of Technology (www.sut.ac.th) [grant number PHD/0098/2553]. She also was supported by Newton Fund from British Council, as well as grants from MY Lab. MY was also supported by the Distinguished Research Professor Grant (NRCT 808/2563) of the National Research Council of Thailand.
Author information
Authors and Affiliations
Contributions
Thitima Sumphanapai performed the experiments, data collection, and writing of the initial draft. Surasak Sawatnatee provided patient samples and had a consultant/advisory role. Jenny Yeung conceived the experiments, supervised assay techniques, and revised the manuscript. Kerry Chester conceived the experiments and provided the critical review, commentary, and revision. Montarop Yamabhai conceived the experiments, conceptualization, supervision, edited the manuscript, and acquisition of the financial support for the project leading to this publication. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The patient samples were collected under the approval of the Ethical Review Board of Sanphasitthiprasong hospital, Ubon Ratchathani, Thailand. The peripheral blood samples of healthy donors were obtained under the approval of the research ethics board of the Suranaree University of Technology, Nakhon Ratchasima, Thailand, in accordance with the Declaration of Helsinki.
Consent to participate
All samples analyzed in this study were obtained after informed consent of the donors.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sumphanapai, T., Chester, K., Sawatnatee, S. et al. Targeting acute myeloid cell surface using a recombinant antibody isolated from whole-cell biopanning of a phage display human scFv antibody library. Med Oncol 39, 205 (2022). https://doi.org/10.1007/s12032-022-01806-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01806-9