Abstract
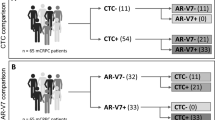
Circulating tumor cells detection and ARV7 expression are associated with worse clinical outcomes in metastatic Castration-Resistant Prostate Cancer (mCRPC) undergoing Androgen Receptor Targeted Agents. ARFL, PSMA and PSA may help to refine prognostic models. In our institution, a prospective observational trial testing CTC detection in mCPRC undergoing I line ARTA therapy terminated the planned enrollment in 2020. Here, we present a pre-planned interim analysis with 18 months of median follow-up. RT-qPCR was used to determine the CTC expression of PSA, PSMA, AR and ARV7 before starting ARTA. PSA-drop, Progression-Free and Overall Survival (PFS and OS) and their correlation with CTC detection were reported. Forty-four patients were included. CTC were detected in 43.2% of patients, of whom 8.94% expressed PSA, 15.78% showed ARV7, 63.15% and 73.68% displayed ARFL and PSMA, respectively. Biochemical response was significantly improved in CTC + vs CTC− patients, with median PSA-drop of 18.5 vs 2.5 ng/ml (p = 0.03). After a median follow-up of 18 months, 50% of patients progressed. PFS was significantly longer in CTC- patients (NR vs 16 months). Eight (18.2%) patients died, a non-significant trend in terms of OS was detected in favor of CTC− patients (NR vs 29 months, p = 0.05). AR, PSA and PSMA expression in CTC + had no significant impact on PSA-drop, PFS or OS. PRIMERA-trial confirmed the CTC detection predictive importance in mCRPC patients.


Similar content being viewed by others
References
Henríquez I, Roach M 3rd, Morgan TM, et al. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines. 2021;9(9):1247. https://doi.org/10.3390/biomedicines9091247.
Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–64. https://doi.org/10.1200/JCO.1996.14.6.1756.
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. https://doi.org/10.1016/S0140-6736(10)61389-X.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. https://doi.org/10.1016/S1470-2045(14)71205-7.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. https://doi.org/10.1056/NEJMoa1405095.
Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–406. https://doi.org/10.1016/S1470-2045(14)70474-7.
Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–103. https://doi.org/10.1056/NEJMoa2107322.
de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102. https://doi.org/10.1056/NEJMoa1911440.
Sweeney C, Bracarda S, Sternberg CN, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398(10295):131–42. https://doi.org/10.1016/S0140-6736(21)00580-8.
Cornford P, Bellmunt J, Bolla M, et al. Guidelines on prostate cancer. part ii: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–42. https://doi.org/10.1016/j.eururo.2016.08.002.
Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149–56. https://doi.org/10.1200/JCO.2016.70.1961.
Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the prophecy study. J Clin Oncol. 2019;37(13):1120–9. https://doi.org/10.1200/JCO.18.01731.
Del Re M, Crucitta S, Sbrana A, et al. AR-V7 and AR-FL expression is associated with clinical outcome: a translational study in patients with castrate resistant prostate cancer. BJU Int. 2019. https://doi.org/10.1111/bju.14792.
Gorges TM, Riethdorf S, von Ahsen O, et al. Heterogeneous PSMA expression on circulating tumor cells: a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget. 2016;7(23):34930–41. https://doi.org/10.18632/oncotarget.9004.
Francolini G, Loi M, Salvestrini V, Mangoni M, Detti B, Di Cataldo V, Aquilano M, Pinzani P, Salvianti F, Desideri I, Mariotti M, Garlatti P, Stocchi G, Ciccone LP, Lucidi S, Salvatore G, Sottili M, Meattini I, Livi L. Prospective assessment of AR splice variant and PSMA detection on circulating tumor cells of mCRPC patients: preliminary analysis of patients enrolled in PRIMERA trial (NCT04188275). Clin Exp Metastasis. 2021;38(5):451–8.
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46. https://doi.org/10.1056/NEJMoa1503747.
Cattrini C, Rubagotti A, Zinoli L, et al. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers (Basel). 2019;11(9):1365. https://doi.org/10.3390/cancers11091365.
Chung JS, Wang Y, Henderson J, et al. Circulating tumor cell-based molecular classifier for predicting resistance to abiraterone and enzalutamide in metastatic castration-resistant prostate cancer. Neoplasia. 2019;21(8):802–9. https://doi.org/10.1016/j.neo.2019.06.002.
Francolini G, Garlatti P, Loi M, Detti B, Aquilano M, Allegra A, Guerrieri B, Salvestrini V, Pinzani P, Bellini C, Salvianti F, Stocchi G, Ciccone L, Salvatore G, Sottili M, Di Cataldo V, Desideri I, Mangoni M, Meattini I, Livi L. ARTO trial (NCT03449719), a randomized phase II trial enrolling oligometastatic castration-resistant prostate cancer patients treated with first-line abiraterone acetate with or without stereotactic body radiation therapy: preliminary results comprehensive of biochemical outcomes and circulating tumor cells analysis. J Clin Oncol. 2021;39(6_suppl):118–118. https://doi.org/10.1200/JCO.2021.39.6_suppl.118.
Cho H, Chung JI, Kim J, et al. Multigene model for predicting metastatic prostate cancer using circulating tumor cells by microfluidic magnetophoresis. Cancer Sci. 2021;112(2):859–70. https://doi.org/10.1111/cas.14745.
Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, Gillessen S, Cook A, Brawley C, Amos CL, Atako N, Pugh C, Buckner M, Chowdhury S, Malik Z, Russell JM, Gilson C, Rush H, Bowen J, Lydon A, Pedley I, O’Sullivan JM, Birtle A, Gale J, Srihari N, Thomas C, Tanguay J, Wagstaff J, Das P, Gray E, Alzoueb M, Parikh O, Robinson A, Syndikus I, Wylie J, Zarkar A, Thalmann G, de Bono JS, Dearnaley DP, Mason MD, Gilbert D, Langley RE, Millman R, Matheson D, Sydes MR, Brown LC, Parmar MKB, James ND, Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–60.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ, ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi T, Shore ND, Rosbrook B, Sugg J, Baron B, Chen L, Stenzl A. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–86.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B, Montesa-Pino Á, Ye D, Parnis F, Cruz F, Tammela TLJ, Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H, Thiele S, Li R, Kuss I, Tombal B, ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–42.
Chi KN, Chowdhury S, Bjartell A, Chung BH, de Santana Pereira, Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–303.
Chi Kim N, Rathkopf Dana E, Smith Matthew Raymond, Efstathiou Eleni, Attard Gerhardt, Olmos David, Lee Ji Youl, Small Eric Jay, Gomes Andrea Juliana, Roubaud Guilhem, Saad Marniza, Zurawski Bogdan, Sakalo Valerii, Mason Gary, del Corral Adam, Wang George C, Daphne Wu, Diorio Brooke, Gitlitz Angela Mennicke Lopez-, Sandhu Shahneen Kaur. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(6):12–12.
Saad Fred, Armstrong Andrew J, Thiery-Vuillemin Antoine, Oya Mototsugu, Loredo Eugenia, Procopio Giuseppe, Janoski Juliana, de Menezes Gustavo, Girotto Colagiovanni, Arslan Cagatay, Mehra Niven, Parnis Francis, Brown Emma, Schlürmann Friederike, Joung Jae Young, Sugimoto Mikio, Poehlein Christian Heinrich, Harrington Elizabeth, Desai Chintu, Kang Jinyu, Clarke Noel. PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40(6_suppl):11–11.
Merseburger AS, Attard G, Boysen G, Gourgioti G, Martins K, Chowdhury S. A randomized, double-blind, placebo (PBO)-controlled, phase 3b study of the efficacy and safety of continuing enzalutamide (ENZA) in chemotherapy-naïve, metastatic castration-resistant prostate cancer (mCRPC) patients (pts) treated with docetaxel (DOC) plus prednisolone (PDN) who have progressed on ENZA: PRESIDE. J Clin Oncol. 2022;40(6_suppl):15–15.
Funding
This study was sponsored under a grant from Ipsen. Ipsen had no input into the study design, analysis or interpretation of results. Ipsen reviewed this manuscript for scientific accuracy but had no input into the content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial or non-financial interests have to be declared.
Ethical appoval
No conflict of interest has to be declared, Protocol was approved by local ethical committee (Comitato Etico Area Vasta Centro).
Informed consent
All patients signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Francolini, G., Loi, M., Ciccone, L.P. et al. Prospective assessment of AR splice variant and multi-biomarker expression on circulating tumor cells of mCRPC patients undergoing androgen receptor targeted agents: interim analysis of PRIMERA trial (NCT04188275). Med Oncol 39, 119 (2022). https://doi.org/10.1007/s12032-022-01756-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01756-2