Abstract
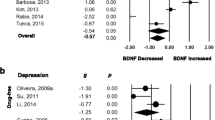
Fibroblast growth factor 2 (FGF2) plays an essential role in neurogenesis, oxidative stress, and emotional behavior. However, the evidence regarding the role of FGF2 in the pathophysiology of depression remains limited and inconclusive. We conducted a systematic review and meta-analysis to investigate peripheral blood FGF2 levels in patients with depression and healthy controls. The PubMed and Web of Science databases were used to identify relevant articles for systematic retrieval. Eight studies involving 310 patients with depression and 268 healthy controls were included in this meta-analysis. A random-effects meta-analysis showed no difference in peripheral blood fibroblast growth factor 2 (FGF2) levels between patients with depression and HC (Hedges’ g = − 0.288, 95% CI = − 0.828 to 0.253, P = 0.297), but there was heterogeneity (Q = 55.719, df = 7, I2 = 87.437, P = 0.000). Subgroup analysis showed no statistically significant differences in the blood (serum/ plasma) and assay (ELISA/ no ELISA) FGF2 levels between all patients with depression and controls; however, there was heterogeneity. The meta-regression analysis showed that age, sex, sample size, depression severity, and publication year did not affect the results. Patients with different subtypes may have mild-to-severe symptoms or a different course of the disease, affecting neurotrophic factor levels. We could not obtain sufficient data from different studies to control for variables. Although the relationship between our findings and the pathophysiology of depression and the role of FGF2 in disease development remains to be determined, FGF2 may be a potential biomarker for depression.





Similar content being viewed by others
Data Availability
Data are available upon request.
References
Akbaba N, Annagür BB, Annagür A, Akbulut H, Akyürek F, Çelık Ç (2018) Neurotrophins and neuroinflammation in fetuses exposed to maternal depression and anxiety disorders during pregnancy: a comparative study on cord blood. Arch Womens Ment Health 21:105–111. https://doi.org/10.1007/s00737-017-0774-1
Assembly WH (2012) Global burden of mental disorders and the need for a comprehensive, coordinated response from health and social workers at the country level: report by the Secretariat. World Health Organization, Geneva, Switzerland
Chen M, Zhang QP, Zhu JX, Cheng J, Liu Q, Xu GH, Li CF, Yi LT (2020) Involvement of FGF-2 modulation in the antidepressant-like effects of liquiritin in mice. Eur J Pharmacol 881, 173297. https://doi.org/10.1016/j.ejphar.2020.173297
Cheng J, Chen M, Wan HQ, Chen XQ, Li CF, Zhu JX, Liu Q, Xu GH, Yi LT (2021) Paeoniflorin exerts antidepressant-like effects through enhancing neuronal FGF-2 by microglial inactivation. J Ethnopharmacol 274:114046. https://doi.org/10.1016/j.jep.2021.114046
Cohen J (1992) A power primer. Psychol Bull 112:155–159. https://doi.org/10.1037//0033-2909.112.1.155
Duman RS, Li N (2012) A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 367:2475–2484. https://doi.org/10.1098/rstb.2011.0357
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. https://doi.org/10.1016/j.biopsych.2006.02.013
Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS (2012) Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry 72:258–265. https://doi.org/10.1016/j.biopsych.2012.03.003
Fournier NM, Duman RS (2012) Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res 227:440–449. https://doi.org/10.1016/j.bbr.2011.04.022
Gaarden TL, Engedal K, Benth JŠ, Larsen M, Lorentzen B, Mollnes TE, Bjølseth TM, Castellheim A (2018) Exploration of 27 plasma immune markers: a cross-sectional comparison of 64 old psychiatric inpatients having unipolar major depression and 18 non-depressed old persons. BMC Geriatr 18:149. https://doi.org/10.1186/s12877-018-0836-x
Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M (2006) Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull 70:221–227. https://doi.org/10.1016/j.brainresbull.2006.04.008
He S, Zhang T, Hong B, Peng D, Su H, Lin Z, Fang Y, Jiang K, Liu X, Li H (2014) Decreased serum fibroblast growth factor - 2 levels in pre- and post-treatment patients with major depressive disorder. Neurosci Lett 579:168–172. https://doi.org/10.1016/j.neulet.2014.07.035
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Hisaoka K, Tsuchioka M, Yano R, Maeda N, Kajitani N, Morioka N, Nakata Y, Takebayashi M (2011) Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J Biol Chem 286:21118–21128. https://doi.org/10.1074/jbc.M111.224683
Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U (2009) Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology 34:353–357. https://doi.org/10.1016/j.psyneuen.2008.09.016
Katsouri L, Ashraf A, Birch AM, Lee KK, Mirzaei N, Sastre M (2015) Systemic administration of fibroblast growth factor-2 (FGF2) reduces BACE1 expression and amyloid pathology in APP23 mice. Neurobiol Aging 36:821–831. https://doi.org/10.1016/j.neurobiolaging.2014.10.004
Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, Patel M, Hodes GE, Russo SJ, Merad M, Iosifescu DV (2017) Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry 7:e1065. https://doi.org/10.1038/tp.2017.31
Kraus C, Kadriu B, Lanzenberger R, Zarate Jr CA, Kasper S (2020) Prognosis and improved outcomes in major depression: a review. Focus Am Psychiatr Publ 18:220–235. https://doi.org/10.1176/appi.focus.18205
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100. https://doi.org/10.1371/journal.pmed.1000100
Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, Wu W, Liao M, Wang M, Tang H, Li W (2013) Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry 54:953–961. https://doi.org/10.1016/j.comppsych.2013.03.026
Qin XY, Cao C, Cawley NX, Liu TT, Yuan J, Loh YP, Cheng Y (2017) Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N = 7277). Mol Psychiatry 22:312–320. https://doi.org/10.1038/mp.2016.62
Reuss B, von Bohlen und Halbach O (2003) Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res 313:139–157. https://doi.org/10.1007/s00441-003-0756-7
Shan X, Chen J, Dai S, Wang J, Huang Z, Lv Z, Wang Q, Wu Q (2020) Cyanidin-related antidepressant-like efficacy requires PI3K/AKT/FoxG1/FGF-2 pathway modulated enhancement of neuronal differentiation and dendritic maturation. Phytomedicine 76:153269. https://doi.org/10.1016/j.phymed.2020.153269
Shi Y, Luan D, Song R, Zhang Z (2020) Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol 41:40–51. https://doi.org/10.1016/j.euroneuro.2020.09.633
Simard S, Shail P, MacGregor J, El Sayed M, Duman RS, Vaccarino FM, Salmaso N (2018) Fibroblast growth factor 2 is necessary for the antidepressant effects of fluoxetine. PLoS One 13:e0204980. https://doi.org/10.1371/journal.pone.0204980
Smith K (2014) Mental health: a world of depression. Nature 515:181. https://doi.org/10.1038/515180a
Takebayashi M, Hashimoto R, Hisaoka K, Tsuchioka M, Kunugi H (2010) Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J Neural Transm (Vienna) 117:1119–1122. https://doi.org/10.1007/s00702-010-0452-1
Turner CA, Watson SJ, Akil H (2012) The fibroblast growth factor family: neuromodulation of affective behavior. Neuron 76:160–174. https://doi.org/10.1016/j.neuron.2012.08.037
Wagner JP, Black IB, DiCicco-Bloom E (1999) Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci 19:6006–6016. https://doi.org/10.1523/jneurosci.19-14-06006.1999
Wang L, Li XX, Chen X, Qin XY, Kardami E, Cheng Y (2018) Antidepressant-like effects of low- and high-molecular weight FGF-2 on chronic unpredictable mild stress mice. Front Mol Neurosci 11:377. https://doi.org/10.3389/fnmol.2018.00377
Wei Z, Li X, Li X, Liu Q, Cheng Y (2018) Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Front Mol Neurosci 11:236. https://doi.org/10.3389/fnmol.2018.00236
Wu CK, Tseng PT, Chen YW, Tu KY, Lin PY (2016) Significantly higher peripheral fibroblast growth factor-2 levels in patients with major depressive disorder: a preliminary meta-analysis under MOOSE guidelines. Medicine (Baltimore) 95:e4563. https://doi.org/10.1097/md.0000000000004563
Wu HE, Teixeira AL, Barroso L, Silva APM, de Souza Nicolau M, Ferreira JDR, Bertola L, Vieira EM, Diniz BS (2019) Epidermal growth factor and fibroblast growth factor-2 circulating levels in elderly with major depressive disorder. Psychiatry Res 272:141–143. https://doi.org/10.1016/j.psychres.2018.12.084
Funding
This study was supported by the National Natural Science Foundation of China (82174085).
Author information
Authors and Affiliations
Contributions
Qing-Shan Liu and Yong Cheng conceived and designed the study. Yang Du and Yan-Li Wang collected the data. Lei-Chen and Yan-Li Wang analyzed and interpreted the data. Yan-Li Wang drafted.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YL., Du, Y., Chen, L. et al. Fibroblast Growth Factor 2 Levels in Patients with Major Depressive Disorder: A Meta-analysis. J Mol Neurosci 73, 95–103 (2023). https://doi.org/10.1007/s12031-023-02101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02101-6