Abstract
Background
Cerebrovascular disorders are an important cause of morbidity and mortality in children. The acute care of a child with an ischemic or hemorrhagic stroke or cerebral sinus venous thrombosis focuses on stabilizing the patient, determining the cause of the insult, and preventing secondary injury. Here, we review the use of both invasive and noninvasive neuromonitoring modalities in the care of pediatric patients with arterial ischemic stroke, nontraumatic intracranial hemorrhage, and cerebral sinus venous thrombosis.
Methods
Narrative review of the literature on neuromonitoring in children with cerebrovascular disorders.
Results
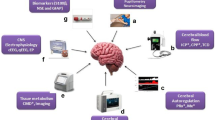
Neuroimaging, near-infrared spectroscopy, transcranial Doppler ultrasonography, continuous and quantitative electroencephalography, invasive intracranial pressure monitoring, and multimodal neuromonitoring may augment the acute care of children with cerebrovascular disorders. Neuromonitoring can play an essential role in the early identification of evolving injury in the aftermath of arterial ischemic stroke, intracranial hemorrhage, or sinus venous thrombosis, including recurrent infarction or infarct expansion, new or recurrent hemorrhage, vasospasm and delayed cerebral ischemia, status epilepticus, and intracranial hypertension, among others, and this, is turn, can facilitate real-time adjustments to treatment plans.
Conclusions
Our understanding of pediatric cerebrovascular disorders has increased dramatically over the past several years, in part due to advances in the neuromonitoring modalities that allow us to better understand these conditions. We are now poised, as a field, to take advantage of advances in neuromonitoring capabilities to determine how best to manage and treat acute cerebrovascular disorders in children.


(Reproduced with permission from Tomko SR, Hahn C, Guerriero RM, Quantitative EEG in the pediatric intensive care unit, in Sansevere AJ, Harrar DB, Atlas of pediatric and neonatal ICU EEG, New York: NY, Demos Medical Publishing. Copyright Springer Publishing Company, LLC.; 2021. p. 363)



(Reproduced with permission from Tomko SR, Hahn C, Guerriero RM, Quantitative EEG in the pediatric intensive care unit, in Sansevere AJ, Harrar DB, Atlas of pediatric and neonatal ICU EEG, New York: NY, Demos Medical Publishing. Copyright Springer Publishing Company, LLC.; 2021. p. 356)
Similar content being viewed by others
References
Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51–96. https://doi.org/10.1161/STR.0000000000000183.
Lehman LL, Khoury JC, Taylor JM, et al. Pediatric stroke rates over 17 years: report from a population-based study. J Child Neurol. 2018;33(7):463–7. https://doi.org/10.1177/0883073818767039.
Steinlin M, Pfister I, Pavlovic J, et al. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36(2):90–7. https://doi.org/10.1055/s-2005-837658.
Christerson S, Strömberg B. Childhood stroke in Sweden I: incidence, symptoms, risk factors and short-term outcome. Acta Paediatr Oslo Nor. 2010;99(11):1641–9. https://doi.org/10.1111/j.1651-2227.2010.01925.x.
Mallick AA, Ganesan V, Kirkham FJ, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014;13(1):35–43. https://doi.org/10.1016/S1474-4422(13)70290-4.
Wintermark M, Hills NK, deVeber GA, et al. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke. 2014;45(12):3597–605. https://doi.org/10.1161/STROKEAHA.114.007404.
Yock-Corrales A, Mackay MT, Mosley I, Maixner W, Babl FE. Acute childhood arterial ischemic and hemorrhagic stroke in the emergency department. Ann Emerg Med. 2011;58(2):156–63. https://doi.org/10.1016/j.annemergmed.2010.10.013.
Simma B, Martin G, Müller T, Huemer M. Risk factors for pediatric stroke: consequences for therapy and quality of life. Pediatr Neurol. 2007;37(2):121–6. https://doi.org/10.1016/j.pediatrneurol.2007.04.005.
deVeber GA, Kirton A, Booth FA, et al. Epidemiology and outcomes of arterial ischemic stroke in children: the Canadian pediatric ischemic stroke registry. Pediatr Neurol. 2017;69:58–70. https://doi.org/10.1016/j.pediatrneurol.2017.01.016.
Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54(2):371–8. https://doi.org/10.1212/wnl.54.2.371.
Goeggel Simonetti B, Cavelti A, Arnold M, et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015;84(19):1941–7. https://doi.org/10.1212/WNL.0000000000001555.
Fox CK, Johnston SC, Sidney S, Fullerton HJ. High critical care usage due to pediatric stroke: results of a population-based study. Neurology. 2012;79(5):420–7. https://doi.org/10.1212/WNL.0b013e3182616fd7.
Mallick AA, Ganesan V, Kirkham FJ, et al. Outcome and recurrence 1 year after pediatric arterial ischemic stroke in a population-based cohort. Ann Neurol. 2016;79(5):784–93. https://doi.org/10.1002/ana.24626.
Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61(2):189–94. https://doi.org/10.1212/01.wnl.0000078894.79866.95.
Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41(2):313–8. https://doi.org/10.1161/STROKEAHA.109.568071.
Livingston JH, Brown JK. Intracerebral haemorrhage after the neonatal period. Arch Dis Child. 1986;61(6):538–44. https://doi.org/10.1136/adc.61.6.538.
Friefeld SJ, Westmacott R, Macgregor D, Deveber GA. Predictors of quality of life in pediatric survivors of arterial ischemic stroke and cerebral sinovenous thrombosis. J Child Neurol. 2011;26(9):1186–92. https://doi.org/10.1177/0883073811408609.
De Schryver ELLM, Blom I, Braun KPJ, et al. Long-term prognosis of cerebral venous sinus thrombosis in childhood. Dev Med Child Neurol. 2004;46(8):514–9. https://doi.org/10.1017/s0012162204000866.
Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. https://doi.org/10.1056/NEJMra072149.
McGlennan C, Ganesan V. Delays in investigation and management of acute arterial ischaemic stroke in children. Dev Med Child Neurol. 2008;50(7):537–40. https://doi.org/10.1111/j.1469-8749.2008.03012.x.
Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124(2):e227-234. https://doi.org/10.1542/peds.2008-3544.
Wycliffe ND, Holshouser BA, Bartnik-Olson B, Ashwal S. Pediatric Neuroimaging. In: Swaiman’s Pediatric Neurology: Principle and Practice. 6th ed.; 2018.
De Jong G, Kannikeswaran N, DeLaroche A, Farooqi A, Sivaswamy L. Rapid sequence MRI Protocol in the evaluation of pediatric brain attacks. Pediatr Neurol. 2020;107:77–83. https://doi.org/10.1016/j.pediatrneurol.2019.12.007.
Mirsky DM, Beslow LA, Amlie-Lefond C, et al. Pathways for neuroimaging of childhood stroke. Pediatr Neurol. 2017;69:11–23. https://doi.org/10.1016/j.pediatrneurol.2016.12.004.
Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10(11):978–86. https://doi.org/10.1016/S1474-4422(11)70192-2.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–22. https://doi.org/10.1056/NEJMoa1804355.
Rafay MF, Armstrong D, Deveber G, Domi T, Chan A, MacGregor DL. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol. 2006;21(1):8–16. https://doi.org/10.1177/08830738060210010101.
Bhatia KD, Briest R, Goetti R, et al. Incidence and natural history of pediatric large vessel occlusion stroke: a population study. JAMA Neurol. 2022. https://doi.org/10.1001/jamaneurol.2022.0323.
Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47(1):273–81. https://doi.org/10.1161/STROKEAHA.115.009171.
Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the american society of neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218–29. https://doi.org/10.3174/ajnr.A4893.
Donahue MJ, Dlamini N, Bhatia A, Jordan LC. Neuroimaging advances in pediatric stroke. Stroke. 2019;50(2):240–8. https://doi.org/10.1161/STROKEAHA.118.020478.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18. https://doi.org/10.1056/NEJMoa1713973.
Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795–803. https://doi.org/10.1056/NEJMoa1813046.
Visser MJ, Yang JYM, Calamante F, et al. Automated perfusion-diffusion magnetic resonance imaging in childhood arterial ischemic stroke. Stroke. 2021;52(10):3296–304. https://doi.org/10.1161/STROKEAHA.120.032822.
Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging JMRI. 2010;32(5):1024–37. https://doi.org/10.1002/jmri.22338.
Lee S, Heit JJ, Albers GW, et al. Neuroimaging selection for thrombectomy in pediatric stroke: a single-center experience. J Neurointerventional Surg. 2019;11(9):940–6. https://doi.org/10.1136/neurintsurg-2019-014862.
Zaharchuk G, El Mogy IS, Fischbein NJ, Albers GW. Comparison of arterial spin labeling and bolus perfusion-weighted imaging for detecting mismatch in acute stroke. Stroke. 2012;43(7):1843–8. https://doi.org/10.1161/STROKEAHA.111.639773.
Huang YC, Liu HL, Lee JD, et al. Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion mri in patients with acute stroke. PLoS ONE. 2013;8(7):e69085. https://doi.org/10.1371/journal.pone.0069085.
Kussman BD, Imaduddin SM, Gharedaghi MH, Heldt T, LaRovere K. Cerebral emboli monitoring using transcranial doppler ultrasonography in adults and children: a review of the current technology and clinical applications in the perioperative and intensive care setting. Anesth Analg. 2021;133(2):379–92. https://doi.org/10.1213/ANE.0000000000005417.
Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. https://doi.org/10.1056/NEJM199807023390102.
Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326(9):605–10. https://doi.org/10.1056/NEJM199202273260905.
Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–48. https://doi.org/10.1001/jama.2014.10517.
DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv. 2020;4(8):1554–88. https://doi.org/10.1182/bloodadvances.2019001142.
Demchuk AM, Christou I, Wein TH, et al. Specific transcranial Doppler flow findings related to the presence and site of arterial occlusion. Stroke. 2000;31(1):140–6. https://doi.org/10.1161/01.str.31.1.140.
Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38(3):948–54. https://doi.org/10.1161/01.STR.0000257304.21967.ba.
Demchuk AM, Burgin WS, Christou I, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32(1):89–93. https://doi.org/10.1161/01.STR.32.1.89.
Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69(1):130–40. https://doi.org/10.1002/ana.22224.
Felling RJ, Sun LR, Maxwell EC, Goldenberg N, Bernard T. Pediatric arterial ischemic stroke: epidemiology, risk factors, and management. Blood Cells Mol Dis. 2017;67:23–33. https://doi.org/10.1016/j.bcmd.2017.03.003.
Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis Basel Switz. 2000;10(6):490–6. https://doi.org/10.1159/000016119.
Koutroulou I, Tsivgoulis G, Tsalikakis D, Karacostas D, Grigoriadis N, Karapanayiotides T. Epidemiology of patent Foramen Ovale in general population and in stroke patients: a narrative review. Front Neurol. 2020;11:281. https://doi.org/10.3389/fneur.2020.00281.
Rubin MN, Alexandrov AV, Douville C, Rinsky B, Tsivgoulis G. Novel robotic TCD ultrasound with bubbles versus standard care to detect right to left shunt: study methods. J Neuroimaging Off J Am Soc Neuroimaging. 2021;31(5):858–63. https://doi.org/10.1111/jon.12890.
Kamouchi M, Kishikawa K, Matsuo R, et al. Ultrasonographic detection of extracranial vertebral artery compression in bow hunter’s brain ischemia caused by neck rotation. Cerebrovasc Dis Basel Switz. 2003;16(3):303–5. https://doi.org/10.1159/000071134.
Regenhardt RW, Kozberg MG, Dmytriw AA, et al. Bow Hunter’s syndrome. Stroke. 2022;53(1):e26–9. https://doi.org/10.1161/STROKEAHA.121.037253.
Marsh EB, Ziai WC, Llinas RH. The need for a rational approach to vasoconstrictive syndromes: transcranial doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol. 2016;8(2):161–71. https://doi.org/10.1159/000447626.
Hathidara M, Patel NH, Flores A, Cabrera Y, Cabrera F, Koch S. Transcranial Doppler findings in reversible cerebral vasoconstriction syndrome. J Neuroimaging Off J Am Soc Neuroimaging. 2022;32(2):345–51. https://doi.org/10.1111/jon.12946.
Cho H, Jo KI, Yu J, Yeon JY, Hong SC, Kim JS. Low flow velocity in the middle cerebral artery predicting infarction after bypass surgery in adult moyamoya disease. J Neurosurg. 2017;126(5):1573–7. https://doi.org/10.3171/2016.3.JNS152256.
Lee YS, Jung KH, Roh JK. Diagnosis of moyamoya disease with transcranial Doppler sonography: correlation study with magnetic resonance angiography. J Neuroimaging Off J Am Soc Neuroimaging. 2004;14(4):319–23. https://doi.org/10.1177/1051228404264958.
Takase K, Kashihara M, Hashimoto T. Transcranial Doppler ultrasonography in patients with moyamoya disease. Clin Neurol Neurosurg. 1997;99(Suppl 2):S101-105. https://doi.org/10.1016/s0303-8467(97)00066-8.
Kwag HJ, Jeong DW, Lee SH, Kim DH, Kim J. Intracranial hemodynamic changes during adult moyamoya disease progression. J Clin Neurol Seoul Korea. 2008;4(2):67–74. https://doi.org/10.3988/jcn.2008.4.2.67.
Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special writing group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39(9):2644–91. https://doi.org/10.1161/STROKEAHA.108.189696.
Gardner Yelton SE, Williams MA, Young M, et al. Perioperative management of pediatric patients with moyamoya arteriopathy. Published online 2021.https://doi.org/10.1055/s-0041-1731667.
LaRovere KL. Transcranial Doppler ultrasound in children with stroke and cerebrovascular disorders. Curr Opin Pediatr. 2015;27(6):712–8. https://doi.org/10.1097/MOP.0000000000000282.
Yang M, Yang Z, Yuan T, Feng W, Wang P. A systemic review of functional near-infrared spectroscopy for stroke: current application and future directions. Front Neurol. 2019;10:58. https://doi.org/10.3389/fneur.2019.00058.
Petersen NH, Silverman A, Strander SM, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51(3):914–21. https://doi.org/10.1161/STROKEAHA.119.026596.
Moreau F, Yang R, Nambiar V, Demchuk AM, Dunn JF. Near-infrared measurements of brain oxygenation in stroke. Neurophotonics. 2016;3(3):031403. https://doi.org/10.1117/1.NPh.3.3.031403.
Ogasawara K, Konno H, Yukawa H, Endo H, Inoue T, Ogawa A. Transcranial regional cerebral oxygen saturation monitoring during carotid endarterectomy as a predictor of postoperative hyperperfusion. Neurosurgery. 2003;53(2):309–14. https://doi.org/10.1227/01.neu.0000073547.86747.f3.
Hansen ML, Hyttel-Sørensen S, Jakobsen JC, et al. Cerebral near-infrared spectroscopy monitoring (NIRS) in children and adults: a systematic review with meta-analysis. Pediatr Res. 2022. https://doi.org/10.1038/s41390-022-01995-z.
Tuckuviene R, Christensen AL, Helgestad J, Johnsen SP, Kristensen SR. Paediatric arterial ischaemic stroke and cerebral sinovenous thrombosis in Denmark 1994–2006: a nationwide population-based study. Acta Paediatr Oslo Nor. 2011;100(4):543–9. https://doi.org/10.1111/j.1651-2227.2010.02100.x.
Hartman AL, Lunney KM, Serena JE. Pediatric stroke: do clinical factors predict delays in presentation? J Pediatr. 2009;154(5):727–32. https://doi.org/10.1016/j.jpeds.2008.11.011.
Billinghurst LL, Beslow LA, Abend NS, et al. Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology. 2017;88(7):630–7. https://doi.org/10.1212/WNL.0000000000003603.
Fox CK, Mackay MT, Dowling MM, et al. Prolonged or recurrent acute seizures after pediatric arterial ischemic stroke are associated with increasing epilepsy risk. Dev Med Child Neurol. 2017;59(1):38–44. https://doi.org/10.1111/dmcn.13198.
Abend NS, Beslow LA, Smith SE, et al. Seizures as a presenting symptom of acute arterial ischemic stroke in childhood. J Pediatr. 2011;159(3):479–83. https://doi.org/10.1016/j.jpeds.2011.02.004.
Singh RK, Zecavati N, Singh J, et al. Seizures in acute childhood stroke. J Pediatr. 2012;160(2):291–6. https://doi.org/10.1016/j.jpeds.2011.07.048.
Song JL, Kim JA, Struck AF, Zhang R, Westover MB. A model of metabolic supply-demand mismatch leading to secondary brain injury. J Neurophysiol. 2021;126(2):653–67. https://doi.org/10.1152/jn.00674.2020.
Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain J Neurol. 2014;137(Pt 5):1429–38. https://doi.org/10.1093/brain/awu042.
Fox CK, Glass HC, Sidney S, Lowenstein DH, Fullerton HJ. Acute seizures predict epilepsy after childhood stroke. Ann Neurol. 2013;74(2):249–56. https://doi.org/10.1002/ana.23916.
Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39(8):1337–51. https://doi.org/10.1007/s00134-013-2938-4.
Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32(2):87–95. https://doi.org/10.1097/WNP.0000000000000166.
Kirschen MP, LaRovere K, Balakrishnan B, et al. A survey of neuromonitoring practices in North American pediatric intensive care units. Pediatr Neurol. 2022;126:125–30. https://doi.org/10.1016/j.pediatrneurol.2021.11.002.
Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63(12):1750–5. https://doi.org/10.1001/archneur.63.12.1750.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–8. https://doi.org/10.1212/01.wnl.0000125184.88797.62.
Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37(3):165–70. https://doi.org/10.1016/j.pediatrneurol.2007.05.012.
Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia. 2010;51(7):1198–204. https://doi.org/10.1111/j.1528-1167.2009.02517.x.
Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–7. https://doi.org/10.1212/WNL.0b013e318211c19e.
Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72(22):1931–40. https://doi.org/10.1212/WNL.0b013e3181a82687.
Fung FW, Jacobwitz M, Parikh DS, et al. Development of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020;61(3):498–508. https://doi.org/10.1111/epi.16448.
Dericioglu N, Yetim E, Bas DF, et al. Non-expert use of quantitative EEG displays for seizure identification in the adult neuro-intensive care unit. Epilepsy Res. 2015;109:48–56. https://doi.org/10.1016/j.eplepsyres.2014.10.013.
Swisher CB, White CR, Mace BE, et al. Diagnostic accuracy of electrographic seizure detection by neurophysiologists and non-neurophysiologists in the adult ICU using a panel of quantitative EEG trends. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32(4):324–30. https://doi.org/10.1097/WNP.0000000000000144.
Topjian AA, Fry M, Jawad AF, et al. Detection of electrographic seizures by critical care providers using color density spectral array after cardiac arrest is feasible. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2015;16(5):461–7. https://doi.org/10.1097/PCC.0000000000000352.
Amorim E, Williamson CA, Moura LMVR, et al. Performance of spectrogram-based seizure identification of adult EEGs by critical care nurses and neurophysiologists. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2017;34(4):359–64. https://doi.org/10.1097/WNP.0000000000000368.
Lalgudi Ganesan S, Stewart CP, Atenafu EG, et al. Seizure identification by critical care providers using quantitative electroencephalography. Crit Care Med. 2018;46(12):e1105–11. https://doi.org/10.1097/CCM.0000000000003385.
Williamson CA, Wahlster S, Shafi MM, Westover MB. Sensitivity of compressed spectral arrays for detecting seizures in acutely ill adults. Neurocrit Care. 2014;20(1):32–9. https://doi.org/10.1007/s12028-013-9912-4.
Du Pont-Thibodeau G, Sanchez SM, Jawad AF, et al. Seizure detection by critical care providers using amplitude-integrated electroencephalography and color density spectral array in pediatric cardiac arrest patients. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(4):363–9. https://doi.org/10.1097/PCC.0000000000001099.
Kang JH, Sherill GC, Sinha SR, Swisher CB. A trial of real-time electrographic seizure detection by neuro-ICU nurses using a panel of quantitative EEG trends. Neurocrit Care. 2019;31(2):312–20. https://doi.org/10.1007/s12028-019-00673-z.
Hofmeijer J, van Putten MJAM. Ischemic cerebral damage: an appraisal of synaptic failure. Stroke. 2012;43(2):607–15. https://doi.org/10.1161/STROKEAHA.111.632943.
Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. Crit Care Lond Engl. 2012;16(2):216. https://doi.org/10.1186/cc11230.
Press CA, Morgan L, Mills M, et al. Spectral electroencephalogram analysis for the evaluation of encephalopathy grade in children with acute liver failure. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(1):64–72. https://doi.org/10.1097/PCC.0000000000001016.
Huguenard AL, Guerriero RM, Tomko SR, et al. Immediate postoperative electroencephalography monitoring in pediatric moyamoya disease and syndrome. Pediatr Neurol. 2021;118:40–5. https://doi.org/10.1016/j.pediatrneurol.2021.02.004.
Sansevere AJ, DiBacco ML, Pearl PL, Rotenberg A. Quantitative electroencephalography for early detection of elevated intracranial pressure in critically Ill children: case series and proposed protocol. J Child Neurol. 2022;37(1):5–11. https://doi.org/10.1177/08830738211015012.
Finnigan SP, Walsh M, Rose SE, Chalk JB. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2007;118(11):2525–32. https://doi.org/10.1016/j.clinph.2007.07.021.
Schleiger E, Sheikh N, Rowland T, Wong A, Read S, Finnigan S. Frontal EEG delta/alpha ratio and screening for post-stroke cognitive deficits: the power of four electrodes. Int J Psychophysiol Off J Int Organ Psychophysiol. 2014;94(1):19–24. https://doi.org/10.1016/j.ijpsycho.2014.06.012.
Leng LZ, Fink ME, Iadecola C. Spreading depolarization: a possible new culprit in the delayed cerebral ischemia of subarachnoid hemorrhage. Arch Neurol. 2011;68(1):31–6. https://doi.org/10.1001/archneurol.2010.226.
Strong AJ, Dardis R. Depolarisation phenomena in traumatic and ischaemic brain injury. Adv Tech Stand Neurosurg. 2005;30:3–49. https://doi.org/10.1007/3-211-27208-9_1.
Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2011;31(1):17–35. https://doi.org/10.1038/jcbfm.2010.191.
Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–47. https://doi.org/10.1038/nm.2333.
Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134(Pt B):189–207. https://doi.org/10.1016/j.neuropharm.2017.09.027.
Hartings JA, Rolli ML, Lu XCM, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci Off J Soc Neurosci. 2003;23(37):11602–10.
Hartings JA, Tortella FC, Rolli ML. AC electrocorticographic correlates of peri-infarct depolarizations during transient focal ischemia and reperfusion. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2006;26(5):696–707. https://doi.org/10.1038/sj.jcbfm.9600223.
Back T, Kohno K, Hossmann KA. Cortical negative DC deflections following middle cerebral artery occlusion and KCl-induced spreading depression: effect on blood flow, tissue oxygenation, and electroencephalogram. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1994;14(1):12–9. https://doi.org/10.1038/jcbfm.1994.3.
Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10(6):754–62. https://doi.org/10.1038/nn1902.
Gyngell ML, Busch E, Schmitz B, et al. Evolution of acute focal cerebral ischaemia in rats observed by localized 1H MRS, diffusion-weighted MRI, and electrophysiological monitoring. NMR Biomed. 1995;8(5):206–14. https://doi.org/10.1002/nbm.1940080505.
Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatr Neurol. 2007;36(2):73–80. https://doi.org/10.1016/j.pediatrneurol.2006.09.017.
Sporns PB, Psychogios MN, Boulouis G, et al. Neuroimaging of acute intracerebral hemorrhage. J Clin Med. 2021;10(5):1086. https://doi.org/10.3390/jcm10051086.
Boulouis G, Blauwblomme T, Hak JF, et al. Nontraumatic pediatric intracerebral hemorrhage. Stroke. 2019;50(12):3654–61. https://doi.org/10.1161/STROKEAHA.119.025783.
Probert R, Saunders DE, Ganesan V. Reversible cerebral vasoconstriction syndrome: rare or underrecognized in children? Dev Med Child Neurol. 2013;55(4):385–9. https://doi.org/10.1111/j.1469-8749.2012.04433.x.
Sari S, Verim S, Hamcan S, et al. MRI diagnosis of dural sinus - Cortical venous thrombosis: Immediate post-contrast 3D GRE T1-weighted imaging versus unenhanced MR venography and conventional MR sequences. Clin Neurol Neurosurg. 2015;134:44–54. https://doi.org/10.1016/j.clineuro.2015.04.013.
Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67(4):518–24. https://doi.org/10.3171/jns.1987.67.4.0518.
Beslow LA, Ichord RN, Gindville MC, et al. Frequency of hematoma expansion after spontaneous intracerebral hemorrhage in children. JAMA Neurol. 2014;71(2):165–71. https://doi.org/10.1001/jamaneurol.2013.4672.
Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–64. https://doi.org/10.1001/jamaneurol.2013.5433.
Schindlbeck KA, Santaella A, Galinovic I, et al. Spot sign in acute intracerebral hemorrhage in dynamic T1-Weighted magnetic resonance imaging. Stroke. 2016;47(2):417–23. https://doi.org/10.1161/STROKEAHA.115.011570.
Selariu E, Zia E, Brizzi M, Abul-Kasim K. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol. 2012;12:109. https://doi.org/10.1186/1471-2377-12-109.
Boulouis G, Morotti A, Brouwers HB, et al. Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol. 2016;73(8):961–8. https://doi.org/10.1001/jamaneurol.2016.1218.
Garg K, Singh PK, Sharma BS, et al. Pediatric intracranial aneurysms–our experience and review of literature. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2014;30(5):873–83. https://doi.org/10.1007/s00381-013-2336-9.
Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37. https://doi.org/10.1161/STR.0b013e3182587839.
Moftakhar P, Cooke DL, Fullerton HJ, et al. Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J Neurosurg Pediatr. 2015;15(3):282–90. https://doi.org/10.3171/2014.9.PEDS14313.
Isola C, Evain JN, Francony G, et al. Cerebral vasospasm in children with subarachnoid hemorrhage: frequency, diagnosis, and therapeutic management. Neurocrit Care. Published online November 17, 2021. doi: https://doi.org/10.1007/s12028-021-01388-w.
Marshall SA, Nyquist P, Ziai WC. The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):291–303. https://doi.org/10.1016/j.nec.2009.10.010.
Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44(6):1237–47.
Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien). 1988;42:81–4. https://doi.org/10.1007/978-3-7091-8975-7_16.
Kirsch JD, Mathur M, Johnson MH, Gowthaman G, Scoutt LM. Advances in transcranial Doppler US: imaging ahead. Radiogr Rev Publ Radiol Soc N Am Inc. 2013;33(1):E1–E14. https://doi.org/10.1148/rg.331125071.
Sun LR, Ziai W, Brown P, et al. Intrathecal chemotherapy-associated cerebral vasospasm in children with hematologic malignancies. Pediatr Res. 2021;89(4):858–62. https://doi.org/10.1038/s41390-020-1008-1.
O’Brien NF, Maa T, Yeates KO. The epidemiology of vasospasm in children with moderate-to-severe traumatic brain injury. Crit Care Med. 2015;43(3):674–85. https://doi.org/10.1097/CCM.0000000000000745.
Zweifel C, Castellani G, Czosnyka M, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41(9):1963–8. https://doi.org/10.1161/STROKEAHA.109.577320.
Park JJ, Kim Y, Chai CL, Jeon JP. Application of near-infrared spectroscopy for the detection of delayed cerebral ischemia in poor-grade subarachnoid hemorrhage. Neurocrit Care. 2021;35(3):767–74. https://doi.org/10.1007/s12028-021-01223-2.
Salonia R, Bell MJ, Kochanek PM, Berger RP. The utility of near infrared spectroscopy in detecting intracranial hemorrhage in children. J Neurotrauma. 2012;29(6):1047–53. https://doi.org/10.1089/neu.2011.1890.
Brogan RJ, Kontojannis V, Garara B, Marcus HJ, Wilson MH. Near-infrared spectroscopy (NIRS) to detect traumatic intracranial haematoma: a systematic review and meta-analysis. Brain Inj. 2017;31(5):581–8. https://doi.org/10.1080/02699052.2017.1287956.
Lin N, Smith ER, Scott RM, Orbach DB. Safety of neuroangiography and embolization in children: complication analysis of 697 consecutive procedures in 394 patients. J Neurosurg Pediatr. 2015;16(4):432–8. https://doi.org/10.3171/2015.2.PEDS14431.
Orbach DB, Stamoulis C, Strauss KJ, et al. Neurointerventions in children: radiation exposure and its import. AJNR Am J Neuroradiol. 2014;35(4):650–6. https://doi.org/10.3174/ajnr.A3758.
Chaudhary N, Elijovich L, Martinez M, et al. Pediatric diagnostic cerebral angiography: practice recommendations from the SNIS Pediatric Committee. J Neurointerventional Surg. 2021;13(8):762–6. https://doi.org/10.1136/neurintsurg-2021-017389.
van Rooij WJ, Jacobs S, Sluzewski M, Beute GN, van der Pol B. Endovascular treatment of ruptured brain AVMs in the acute phase of hemorrhage. AJNR Am J Neuroradiol. 2012;33(6):1162–6. https://doi.org/10.3174/ajnr.A2995.
Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89(4):539–46. https://doi.org/10.3171/jns.1998.89.4.0539.
Brown RD, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73(6):859–63. https://doi.org/10.3171/jns.1990.73.6.0859.
e Sa MJC, Stein BM, Solomon RA, McCormick PC. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77(6):853–9.
Deruty R, Mottolese C, Soustiel JF, Pelissou-Guyotat I. Association of cerebral arteriovenous malformation and cerebral aneurysm. Diagn Manag Acta Neurochir (Wien). 1990;107(3–4):133–9. https://doi.org/10.1007/BF01405792.
Lasjaunias P, Piske R, Terbrugge K, Willinsky R. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA). Analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien). 1988;91(1–2):29–36. https://doi.org/10.1007/BF01400524.
Miyasaka K, Wolpert SM, Prager RJ. The association of cerebral aneurysms, infundibula, and intracranial arteriovenous malformations. Stroke. 1982;13(2):196–203. https://doi.org/10.1161/01.str.13.2.196.
Suzuki J, Onuma T. Intracranial aneurysms associated with arteriovenous malformations. J Neurosurg. 1979;50(6):742–6. https://doi.org/10.3171/jns.1979.50.6.0742.
Turjman F, Massoud TF, Viñuela F, Sayre JW, Guglielmi G, Duckwiler G. Aneurysms related to cerebral arteriovenous malformations: superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol. 1994;15(9):1601–5.
Brown RD, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68(3):352–7. https://doi.org/10.3171/jns.1988.68.3.0352.
Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid haemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25(4):467–90. https://doi.org/10.3171/jns.1966.25.4.0467.
da Costa L, Wallace MC, Ter Brugge KG, O’Kelly C, Willinsky RA, Tymianski M. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40(1):100–5. https://doi.org/10.1161/STROKEAHA.108.524678.
Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350–5. https://doi.org/10.1212/01.wnl.0000210524.68507.87.
Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL, MARS Coinvestigators. Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology. 2014;83(7):590–7. https://doi.org/10.1212/WNL.0000000000000688.
Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29(5):931–4. https://doi.org/10.1161/01.str.29.5.931.
Miyasaka Y, Yada K, Ohwada T, Kitahara T, Kurata A, Irikura K. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76(2):239–43. https://doi.org/10.3171/jns.1992.76.2.0239.
Kader A, Young WL, Pile-Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34(5):801–7. https://doi.org/10.1227/00006123-199405000-00003.
Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18(1):29–35. https://doi.org/10.1227/00006123-198601000-00006.
Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27(1):1–6. https://doi.org/10.1161/01.str.27.1.1.
Forster DM, Steiner L, Håkanson S. Arteriovenous malformations of the brain. A long-term clinical study. J Neurosurg. 1972;37(5):562–70. https://doi.org/10.3171/jns.1972.37.5.0562.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. https://doi.org/10.1227/00006123-198001000-00001.
Gurusinghe NT, Richardson AE. The value of computerized tomography in aneurysmal subarachnoid haemorrhage. The concept of the CT score. J Neurosurg. 1984;60(4):763–70. https://doi.org/10.3171/jns.1984.60.4.0763.
Qureshi AI, Sung GY, Razumovsky AY, Lane K, Straw RN, Ulatowski JA. Early identification of patients at risk for symptomatic vasospasm after aneurysmal subarachnoid hemorrhage. Crit Care Med. 2000;28(4):984–90. https://doi.org/10.1097/00003246-200004000-00012.
Davis JM, Davis KR, Crowell RM. Subarachnoid hemorrhage secondary to ruptured intracranial aneurysm: prognostic significance of cranial CT. AJR Am J Roentgenol. 1980;134(4):711–5. https://doi.org/10.2214/ajr.134.4.711.
Suzuki J, Komatsu S, Sato T, Sakurai Y. Correlation between CT findings and subsequent development of cerebral infarction due to vasospasm in subarachnoid haemorrhage. Acta Neurochir (Wien). 1980;55(1–2):63–70. https://doi.org/10.1007/BF01808921.
Pasqualin A, Rosta L, Da Pian R, Cavazzani P, Scienza R. Role of computed tomography in the management of vasospasm after subarachnoid hemorrhage. Neurosurgery. 1984;15(3):344–53. https://doi.org/10.1227/00006123-198409000-00009.
Li K, Barras CD, Chandra RV, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;126:513–27. https://doi.org/10.1016/j.wneu.2019.03.083.
Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47(9):1510–8. https://doi.org/10.1111/j.1528-1167.2006.00624.x.
Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81(4):383–91. https://doi.org/10.1212/WNL.0b013e31829c5cfe.
Beslow LA, Abend NS, Gindville MC, et al. Pediatric intracerebral hemorrhage: acute symptomatic seizures and epilepsy. JAMA Neurol. 2013;70(4):448–54. https://doi.org/10.1001/jamaneurol.2013.1033.
Lo WD, Lee J, Rusin J, Perkins E, Roach ES. Intracranial hemorrhage in children: an evolving spectrum. Arch Neurol. 2008;65(12):1629–33. https://doi.org/10.1001/archneurol.2008.502.
Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15(5):284–9. https://doi.org/10.1177/088307380001500503.
Blom I, De Schryver ELLM, Kappelle LJ, Rinkel GJE, Jennekens-Schinkel A, Peters ACB. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2003;45(4):233–9. https://doi.org/10.1017/s001216220300046x.
Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40(5):1666–71. https://doi.org/10.1161/STROKEAHA.108.541383.
Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25(6):416–21. https://doi.org/10.1016/s0387-7604(03)00029-9.
Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48(11):1343–8. https://doi.org/10.1016/0895-4356(95)00039-9.
Yang JS, Park YD, Hartlage PL. Seizures associated with stroke in childhood. Pediatr Neurol. 1995;12(2):136–8. https://doi.org/10.1016/0887-8994(94)00152-r.
Chadehumbe MA, Khatri P, Khoury JC, et al. Seizures are common in the acute setting of childhood stroke: a population-based study. J Child Neurol. 2009;24(1):9–12. https://doi.org/10.1177/0883073808320756.
De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86(3):253–60. https://doi.org/10.1212/WNL.0000000000002281.
Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2004;115(12):2699–710. https://doi.org/10.1016/j.clinph.2004.06.017.
Rots ML, van Putten MJAM, Hoedemaekers CWE, Horn J. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2016;24(2):207–16. https://doi.org/10.1007/s12028-015-0205-y.
Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103(6):607–15. https://doi.org/10.1016/s0013-4694(97)00071-0.
Yu Z, Wen D, Zheng J, et al. Predictive accuracy of alpha-delta ratio on quantitative electroencephalography for delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: meta-analysis. World Neurosurg. 2019;126:e510–6. https://doi.org/10.1016/j.wneu.2019.02.082.
Appavu BL, Temkit MH, Foldes ST, et al. Quantitative electroencephalography after pediatric anterior circulation stroke. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2020. https://doi.org/10.1097/WNP.0000000000000813.
Walker CT, Stone JJ, Jacobson M, Phillips V, Silberstein HJ. Indications for pediatric external ventricular drain placement and risk factors for conversion to a ventriculoperitoneal shunt. Pediatr Neurosurg. 2012;48(6):342–7. https://doi.org/10.1159/000353608.
Waziri A, Claassen J, Stuart RM, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66(3):366–77. https://doi.org/10.1002/ana.21721.
Appavu B, Foldes S, Burrows BT, et al. Multimodal assessment of cerebral autoregulation and autonomic function after pediatric cerebral arteriovenous malformation rupture. Neurocrit Care. 2021;34(2):537–46. https://doi.org/10.1007/s12028-020-01058-3.
Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010;21(3–5):511–27. https://doi.org/10.1016/j.nec.2010.03.006.
Ropper AH, Klein JP. Cerebral venous thrombosis. N Engl J Med. 2021;385(1):59–64. https://doi.org/10.1056/NEJMra2106545.
Bidar F, Faeghi F, Ghorbani A. Assessment of cerebral venous sinus thrombosis using T2*-weighted gradient echo magnetic resonance imaging sequences. Iran J Neurol. 2016;15(2):96–9.
Selim M, Fink J, Linfante I, Kumar S, Schlaug G, Caplan LR. Diagnosis of cerebral venous thrombosis with echo-planar T2*-weighted magnetic resonance imaging. Arch Neurol. 2002;59(6):1021–6. https://doi.org/10.1001/archneur.59.6.1021.
deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345(6):417–23. https://doi.org/10.1056/NEJM200108093450604.
Barnes C, Newall F, Furmedge J, Mackay M, Monagle P. Cerebral sinus venous thrombosis in children. J Paediatr Child Health. 2004;40(1–2):53–5. https://doi.org/10.1111/j.1440-1754.2004.00291.x.
Barron TF, Gusnard DA, Zimmerman RA, Clancy RR. Cerebral venous thrombosis in neonates and children. Pediatr Neurol. 1992;8(2):112–6. https://doi.org/10.1016/0887-8994(92)90030-3.
Zhu X, Liu M, Gong X, et al. Transcranial color-coded sonography for the detection of cerebral veins and sinuses and diagnosis of cerebral venous sinus thrombosis. Ultrasound Med Biol. 2019;45(10):2649–57. https://doi.org/10.1016/j.ultrasmedbio.2019.06.419.
Ichord RN, Benedict SL, Chan AK, Kirkham FJ, Nowak-Göttl U. Paediatric cerebral sinovenous thrombosis: findings of the International Paediatric stroke study. Arch Dis Child. 2015;100(2):174–9. https://doi.org/10.1136/archdischild-2014-306382.
Munjal NK, Bergman I, Scheuer ML, Genovese CR, Simon DW, Patterson CM. Quantitative electroencephalography (EEG) predicting acute neurologic deterioration in the pediatric intensive care unit: a case series. J Child Neurol. 2022;37(1):73–9. https://doi.org/10.1177/08830738211053908.
Havalı C, İnce H, Gündoğdu EB, et al. The management of elevated intracranial pressure and sinus vein thrombosis associated with mastoiditis: the experience of eighteen patients. Childs Nerv Syst. 2022;38(2):421–8. https://doi.org/10.1007/s00381-021-05402-6.
Armonda RA, Vo AH, Bell R, Neal C, Campbell WW. Multimodal monitoring during emergency hemicraniectomy for vein of Labbe thrombosis. Neurocrit Care. 2006;4(3):241–4. https://doi.org/10.1385/NCC:4:3:241.
Simonin A, Rusca M, Saliou G, Levivier M, Daniel RT, Oddo M. Multimodal regional brain monitoring of tissue ischemia in severe cerebral venous sinus thrombosis. Neurocrit Care. 2019;31(2):297–303. https://doi.org/10.1007/s12028-019-00695-7.
Funding
There was no funding for this article.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data: HDB, SLR, and SAJ. Substantial contributions to acquisition of data and analysis and interpretation of data: SJB and LS. Drafting the article and revising it critically for important intellectual content: HDB, SLR, SJB, and LS. Revising the article critically for important intellectual content: SAJ. Final approval of the version to be published: HDB, SLR, SJB, LS, and SAJ. Agreement to be accountable for all aspects of the work: HDB, SLR, SJB, LS, and SAJ.
Corresponding author
Ethics declarations
Conflict of interest
DH and AJS receive royalties from Springer Publishing Company, LLC for: Sansevere AJ, Harrar DB, eds. Atlas of pediatric and neonatal intensive care unit EEG, New York: NY, Demos Medical Publishing. Copyright Springer Publishing Company, LLC.; 2021. LRS is supported by a career development award from the American Heart Association: Clinical, Radiographic, and Hemodynamic Predictors of Childhood Moyamoya Arteriopathy Progression and Surgical Risk. The remaining authors (JBS, SL) have no conflicts of interest to disclose.
Ethical Approval/Informed Consent
This article adheres to ethical guidelines. Ethical approvals were not sought given that this is an invited review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harrar, D.B., Sun, L.R., Segal, J.B. et al. Neuromonitoring in Children with Cerebrovascular Disorders. Neurocrit Care 38, 486–503 (2023). https://doi.org/10.1007/s12028-023-01689-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01689-2