Abstract
Background
Long-term bed rest in neurointensive care (NIC) patients leads to skeletal muscle atrophy and cognitive dysfunction, which seriously affects the physical fitness and final prognosis of critically ill patients. Exercise therapy plays an increasingly important role in the treatment and rehabilitation of patients with sarcopenia. However, the therapeutic effect and mechanism of exercise therapy for patients with neurological impairment remain unclear.
Methods
Serum samples of NIC patients before and after exercise therapy and normal people were collected to detect interleukin-6 (IL-6) and interleukin-1β levels by enzyme-linked immunosorbent assay (ELISA). Middle cerebral artery occlusion (MCAO) was used for the construction of a rat model. The Morris water maze test, exploration test, and open-field test were used to assess neurological function in rats. Western blot and quantitative real-time polymerase chain reaction were performed to evaluate the activation of IL-6/adenosine-monophosphate-activated protein kinase (AMPK) signaling.
Results
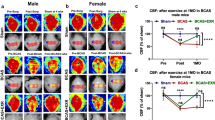
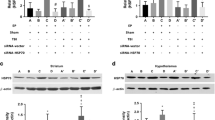
Exercise therapy attenuated IL-6 expression in NIC patients. Exercise therapy alleviated cognitive dysfunctions and decreased IL-6 expression in MCAO rats. Exercise therapy alleviated gastrocnemius muscle injury in rats after MCAO by modulating IL-6/AMPK signaling.
Conclusions
Treadmill exercise decreases inflammation in MCAO rats via modulating IL-6/AMPK signaling.





Similar content being viewed by others
References
Robba C, Wong A, Poole D, et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med. 2021;47:1347–67.
Beckham JD, Tyler KL. Neuro-intensive care of patients with acute CNS infections. Neurotherapeutics. 2012;9:124–38.
Gomes MJ, Martinez PF, Pagan LU, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8:20428–40.
von Haehling S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc. 2015;74:367–77.
Souza RW, Piedade WP, Soares LC, et al. Aerobic exercise training prevents heart failure-induced skeletal muscle atrophy by anti-catabolic, but not anabolic actions. PLoS ONE. 2014;9: e110020.
Tzanis G, Philippou A, Karatzanos E, et al. Effects of high-intensity interval exercise training on skeletal myopathy of chronic heart failure. J Card Fail. 2017;23:36–46.
Cai M, Wang Q, Liu Z, et al. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018;213:40–9.
Lenk K, Erbs S, Hollriegel R, et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19:404–11.
Ribeiro-Samora GA, Rabelo LA, Ferreira ACC, et al. Inflammation and oxidative stress in heart failure: effects of exercise intensity and duration. Braz J Med Biol Res. 2017;50: e6393.
Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–7.
Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 1985;2005(98):557–64.
Nakashima K, Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem. 2007;71:1650–6.
Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412.
Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–6.
Haymore JB, Patel N. Delirium in the neuro intensive care unit. Crit Care Nurs Clin North Am. 2016;28:21–35.
Hutchens MP, Memtsoudis S, Sadovnikoff N. Propofol for sedation in neuro-intensive care. Neurocrit Care. 2006;4:54–62.
Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103–15.
Pincherle A, Johr J, Pancini L, et al. Intensive care admission and early neuro-rehabilitation. Lessons for COVID-19? Front Neurol. 2020;11:880.
Soares MN, Eggelbusch M, Naddaf E, et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J Cachexia Sarcopenia Muscle. 2022;13:11–22.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35.
McArthur S, Juban G, Gobbetti T, et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J Clin Invest. 2020;130:1156–67.
Thomson DM. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int J Mol Sci. 2018;19:3125.
Chen X, Guo Y, Jia G, et al. Arginine promotes skeletal muscle fiber type transformation from fast-twitch to slow-twitch via Sirt1/AMPK pathway. J Nutr Biochem. 2018;61:155–62.
Choi S, Jeong HJ, Kim H, et al. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy. 2019;15:1069–81.
Fan J, Yang X, Li J, et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. 2017;8:17475–90.
Morris BJ, Willcox DC, Donlon TA, et al. FOXO3: a major gene for human longevity–a mini-review. Gerontology. 2015;61:515–25.
Yao S, Fan LY, Lam EW. The FOXO3-FOXM1 axis: a key cancer drug target and a modulator of cancer drug resistance. Semin Cancer Biol. 2018;50:77–89.
Mammucari C, Milan G, Romanello V, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71.
Fitzwalter BE, Thorburn A. FOXO3 links autophagy to apoptosis. Autophagy. 2018;14:1467–8.
Schiaffino S, Dyar KA, Ciciliot S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–314.
Skurk C, Izumiya Y, Maatz H, et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–23.
Raue U, Slivka D, Jemiolo B, et al. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–12.
Léger B, Cartoni R, Praz M, et al. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576:923–33.
Stefanetti RJ, Voisin S, Russell A, et al. Recent advances in understanding the role of FOXO3. F1000Res. 2018;7:1372.
Funding
None.
Author information
Authors and Affiliations
Contributions
HF, YQ, XW, FC, and XL did the experiments, collected and analyzed the data, and wrote the manuscript. HF and XL conceived and coordinated the study.
Corresponding author
Ethics declarations
Conflict of interest
Hui Feng, Yinliang Qi, Xinlong Wang, Fangyu Chen, and Xueping Li declare that they have no competing interest.
Ethical Approval
The study was approved by the Ethics Committee of The Affiliated Jiangning Hospital of Nanjing Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, H., Qi, Y., Wang, X. et al. Treadmill Exercise Decreases Inflammation Via Modulating IL-6 Expression in the Rat Model of Middle Cerebral Artery Occlusion. Neurocrit Care 38, 279–287 (2023). https://doi.org/10.1007/s12028-022-01575-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01575-3