Abstract
Background
Nonconvulsive status epilepticus (NCSE) is a frequent disorder in neurocritical care and diagnosing it can be challenging. NCSE patients often show altered pupil function, but nature and extent may vary. Infrared pupillometry allows detection of subtle changes of pupil function. The neurological pupil index (NPi) is considered a surrogate marker of global pupil function which is supposed to be independent of absolute parameters such as the pupil diameter.
Objective
Cross-sectional observational study to assess whether NPi is altered in NCSE.
Methods
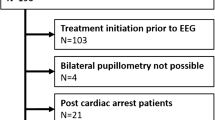
128 consecutive adult emergency patients who had experienced a suspected seizure, have not reached their prior functional level regarding level of consciousness, mental status or focal deficits, had no obvious clinical signs of status epilepticus and had an EEG indication as determined by the treating clinician for exclusion of NCSE were examined by routine EEG and pupillometry. Exclusion criteria were ocular comorbidity (n = 21) and poor EEG quality (n = 4). Pupillometry was performed once directly before the beginning of EEG recording. NCSE diagnosis (no NCSE, possible NCSE and confirmed NCSE) was established according to Salzburg consensus criteria blinded to pupillometry results. Group comparison was performed for right NPi, left NPi, lowest NPi of both sides (minNPi) and the absolute difference of both sides (diffNPi) applying non-parametric testing. In post-hoc analysis, receiver operating characteristics (ROC) of NCSE diagnosis (combined confirmed NCSE and possible NCSE) were performed for minNPi and diffNPi.
Results
From 103 patients included in the final analysis, 5 (4.9%) had confirmed NCSE, 7 (6.8%) had possible NCSE. Right NPi (p = 0.002), left NPi (p < 0.001) and minNPi (p < 0.001) were significantly lower in “confirmed NCSE” and “possible NCSE” compared to “no NCSE”; diffNPi was significantly higher in “confirmed NCSE” and “possible NCSE” compared to “no NCSE” (p < 0.001). There was no significant difference of minNPi and diffNPi between “confirmed NCSE” and “possible NCSE”. ROC analysis showed an optimal cut-off of minNPi for NCSE diagnosis of 4.0 (AUC = 0.93, 95% CI 0.86–0.99). Optimal ROC analysis cut-off of diffNPi for NCSE diagnosis was 0.2 (AUC = 0.89, 95% CI 0.80–0.99).
Conclusions
NPi was significantly reduced and the difference between left and right NPi was significantly higher in confirmed NCSE. An NPi < 4.0 on either side as well as an NPi difference of both sides > 0.2 may be potential indicators of NCSE. Infrared pupillometry may be a helpful diagnostic tool in the assessment of NCSE and should be studied further in larger populations.


Similar content being viewed by others
References
Meierkord H, Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007;6(4):329–39.
Rohracher A, et al. Status epilepticus in the elderly-a retrospective study on 120 patients. Epilepsy Res. 2016;127:317–23.
Beniczky S, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(Suppl 6):28–9.
Leitinger M, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol. 2016;15(10):1054–62.
Couret D, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care. 2016;20:99.
Anderson M, et al. Integrating quantitative pupillometry into regular care in a neurotrauma intensive care unit. J Neurosci Nurs. 2018;50(1):30–6.
Olson DM, Fishel M. The use of automated pupillometry in critical care. Crit Care Nurs Clin N Am. 2016;28(1):101–7.
Olson DM, et al. Interrater reliability of pupillary assessments. Neurocrit Care. 2016;24(2):251–7.
Ong C, Hutch M, Smirnakis S. The effect of ambient light conditions on quantitative pupillometry. Neurocrit Care. 2018;30:316–21.
Elmer J. Quantitative pupillometry after cardiac arrest. Resuscitation. 2018;131:A1–2.
Heimburger D, et al. Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation. 2016;103:88–93.
Solari D, et al. Early prediction of coma recovery after cardiac arrest with blinded pupillometry. Ann Neurol. 2017;81(6):804–10.
Chen JW, et al. Infrared pupillometry, the neurological pupil index and unilateral pupillary dilation after traumatic brain injury: implications for treatment paradigms. Springerplus. 2014;3:548.
Natzeder S, et al. Portable infrared pupillometer in patients with subarachnoid hemorrhage: prognostic value and circadian rhythm of the neurological pupil index (NPi). J Neurosurg Anesthesiol. 2018;31:428–33.
Soeken TA, et al. Quantitative pupillometry for detection of intracranial pressure changes during head-down tilt. Aerosp Med Hum Perform. 2018;89(8):717–23.
Yang E, et al. Infrared pupillometry helps to detect and predict delirium in the post-anesthesia care unit. J Clin Monit Comput. 2018;32(2):359–68.
Laurenzo SA, et al. Pupillary response abnormalities in depressive disorders. Psychiatry Res. 2016;246:492–9.
Mestanikova A, et al. Pupillary light reflex is altered in adolescent depression. Physiol Res. 2017;66(Supplementum 2):S277–84.
Cortez MM, et al. Altered pupillary light response scales with disease severity in migrainous photophobia. Cephalalgia. 2017;37(8):801–11.
Frost S, et al. Evaluation of cholinergic deficiency in preclinical Alzheimer’s disease using pupillometry. J Ophthalmol. 2017;2017:7935406.
Van Stavern GP, et al. Pupillary light reaction in preclinical Alzheimer’s disease subjects compared with normal ageing controls. Br J Ophthalmol. 2018;103:971–5.
Bartošová O, et al. Pupillometry as an indicator of L-DOPA dosages in Parkinson’s disease patients. J Neural Transm (Vienna). 2018;125(4):699–703.
Aydogmus Y, et al. Is overactive bladder a nervous or bladder disorder? Autonomic imaging in patients with overactive bladder via dynamic pupillometry. World J Urol. 2017;35(3):467–72.
Larsen RS, Waters J. Neuromodulatory correlates of pupil dilation. Front Neural Circuits. 2018;12:21.
Shirozu K, et al. The relationship between seizure in electroconvulsive therapy and pupillary response using an automated pupilometer. J Anesth. 2018;32(6):866–71.
Centeno M, et al. Epilepsy causing pupillary hippus: an unusual semiology. Epilepsia. 2011;52(8):e93–6.
Nagayama M, et al. Novel clinical features of nonconvulsive status epilepticus. F1000Res. 2017;6:1690.
Shirozu K, Murayama K, Yamaura K. Pupillary response as assessment of effective seizure induction by electroconvulsive therapy. J Vis Exp. 2019;146:e59488.
Zehtabchi S, et al. Prevalence of non-convulsive seizure and other electroencephalographic abnormalities in ED patients with altered mental status. Am J Emerg Med. 2013;31(11):1578–82.
Firosh Khan S, et al. Emergent EEG is helpful in neurology critical care practice. Clin Neurophysiol. 2005;116(10):2454–9.
Braun M, et al. Coma of unknown origin in the emergency department: implementation of an in-house management routine. Scand J Trauma Resusc Emerg Med. 2016;24:61.
Martindale JL, Goldstein JN, Pallin DJ. Emergency department seizure epidemiology. Emerg Med Clin N Am. 2011;29(1):15–27.
Tiamkao S, et al. Seizure presenting to the emergency department, Srinagarind Hospital. J Med Assoc Thail. 2006;89(3):362–7.
Burkhouse KL, et al. Pupillary reactivity to sad stimuli as a biomarker of depression risk: evidence from a prospective study of children. J Abnorm Psychol. 2015;124(3):498–506.
Kang O, Wheatley T. Pupil dilation patterns reflect the contents of consciousness. Conscious Cogn. 2015;35:128–35.
Sharma S, et al. Factors influencing the pupillary light reflex in healthy individuals. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1353–9.
Schwalm M, Rosales Jubal E. Back to pupillometry: how cortical network state fluctuations tracked by pupil dynamics could explain neural signal variability in human cognitive neuroscience. eNeuro. 2017;4(6).
Elman JA, et al. Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex. 2017;97:60–9.
Zekveld AA, Koelewijn T, Kramer SE. The pupil dilation response to auditory stimuli: current state of knowledge. Trends Hear. 2018;22:2331216518777174.
Unsworth N, Robison MK. Tracking arousal state and mind wandering with pupillometry. Cogn Affect Behav Neurosci. 2018;18(4):638–64.
Miller NR, Walsh FB, Hoyt WF. Walsh and Hoyt’s clinical neuro-ophthalmology. Baltimore, MD: Lippincott Williams & Wilkins; 2005.
Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004;4(2):43–6.
Fernández-Torre JL, et al. Pupillary hippus as clinical manifestation of refractory autonomic nonconvulsive status epilepticus: pathophysiological implications. Seizure. 2018;63:102–4.
Sadek AR, et al. Seizure-induced miosis. Epilepsia. 2011;52(12):e199-203.
Schnell D, et al. Pupillary hippus in nonconvulsive status epilepticus. Epileptic Disord. 2012;14(3):310–2.
Turnbull PR, et al. Origins of pupillary hippus in the autonomic nervous system. Investig Ophthalmol Vis Sci. 2017;58(1):197–203.
Kellinghaus C, et al. Sustained effort network for treatment of status epilepticus (SENSE)—a multicenter prospective observational registry. Epilepsy Behav. 2019;101(Pt B):106553.
Koren J, et al. Prediction of rhythmic and periodic EEG patterns and seizures on continuous EEG with early epileptiform discharges. Epilepsy Behav. 2015;49:286–9.
Hantus S. Monitoring for seizures in the intensive care unit. In: Levin KH, Chauvel P, editors. Handbook of clinical neurology, vol. 161. Amsterdam: Elsevier; 2019. p. 103–7.
Florea B, et al. Semiology of subtle motor phenomena in critically ill patients. Seizure. 2017;48:33–5.
Truong JQ, Ciuffreda KJ. Comparison of pupillary dynamics to light in the mild traumatic brain injury (mTBI) and normal populations. Brain Inj. 2016;30(11):1378–89.
Lussier BL, Olson DM, Aiyagari V. Automated pupillometry in neurocritical care: research and practice. Curr Neurol Neurosci Rep. 2019;19(10):71.
Vinciguerra L, Bösel J. Noninvasive neuromonitoring: current utility in subarachnoid hemorrhage, traumatic brain injury, and stroke. Neurocrit Care. 2017;27(1):122–40.
Funding
None.
Author information
Authors and Affiliations
Contributions
Authorship requirements have been met by all authors. JG, JR and JB designed the study. JG, JR and CB participated in data acquisition. JG wrote up the first draft of the manuscript. All authors took part in data analysis, interpretation and critical review of the manuscript. The final draft of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
JG has nothing to disclose. CB has nothing to disclose. JR reports personal fees from Eisai GmbH, outside the submitted work. JB reports personal fees from Medtronic, personal fees from Boehringer Ingelheim, personal fees from Zoll and grants from PCORI, all outside the submitted work.
Ethical Approval/Informed Consent
The study was performed in adherence to ethical guidelines. Ethical approval including a formal consent waiver for observational pupillometry was granted by the Hesse Medical Association Ethical Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Godau, J., Bierwirth, C., Rösche, J. et al. Quantitative Infrared Pupillometry in Nonconvulsive Status Epilepticus. Neurocrit Care 35, 113–120 (2021). https://doi.org/10.1007/s12028-020-01149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01149-1