Abstract
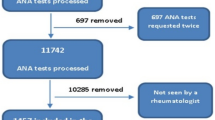
The diagnosis of systemic autoimmune diseases (SAID) is challenging, due to overlapping features with other non-immune disorders. Anti-nuclear antibodies (ANA)/anti-cellular antibodies are the sensitive screening tests but anti-double-stranded-deoxyribonucleic acid-antibody (anti-ds-DNA) and ANA-specific antibodies are specific for SAID. We aimed to look at ANA-specific antibodies in our patients and correlated them with ANA patterns, anti-ds-DNA, and clinical diagnosis for proper interpretation and better patient management cost-effectively. A retrospective data analysis of 641 patients was done (1st of February 2019 to 31st of July 2021) who were tested for ANA-specific antibodies at the Immunology Department of Indus Hospital and Health Network. ANA and anti-ds-DNA results and clinical diagnosis were also analyzed for ANA-specific antibody-positive patients. Descriptive data were presented in mean ± standard deviation and frequency percentages whereas inferential data were analyzed with a chi-square test for association between ANA-specific antibodies status, ANA, anti-ds-DNA, and clinical features. ANA-specific antibodies test revealed positivity for at least one autoantibody in 245 (38.2%) patients. Of these, ANA was tested in 206 patients reactive for ANA-specific antibodies and found positive in 195 (95%) as compared to negative (< 0.001). Speckled and homogenous were predominant ANA patterns in ANA-specific antibody-positives (56% and 42% respectively). Multiple ANA patterns were found in 18 patients most commonly with systemic lupus erythematosus (SLE) and mixed connective tissue disorder (MCTD). Anti-SSA were the most common ANA-specific antibodies (50%) and were mostly found in sera with speckled (61/97) and homogenous (38/97) patterns and associated mostly with SLE (48%) and Sjogren’s syndrome (86%). Among ANA-negative patients, anti-SSA were the most common antibodies (n = 5). Anti-ds-DNA was found in 66% of SLE patients along with another ANA-specific antibody. This study showed that testing for ANA-specific antibodies cannot be gated on ANA patterns. Also, there is a redundancy of these antibodies with various clinical diagnoses. Moreover, they are useful in making a diagnosis in ANA-negative patients as well with clinical suspicion.


Similar content being viewed by others
References
Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63–80. https://doi.org/10.1016/j.cell.2020.03.007.
Missoum H, Alami M, Bachir F, Arji N, Bouyahya A, Rhajaoui M, El Aouad R, Bakri Y. Prevalence of autoimmune diseases and clinical significance of autoantibody profile: data from National Institute of Hygiene in Rabat. Morocco Hum Immunol. 2019;80(7):523–32. https://doi.org/10.1016/j.humimm.2019.02.012.
Avery TY, van de Cruys M, Austen J, Stals F, Damoiseaux JG. Anti-nuclear antibodies in daily clinical practice: prevalence in primary, secondary, and tertiary care. J Immunol Res. 2014;2014: 401739. https://doi.org/10.1155/2014/401739.
Banhuk FW, Pahim BC, Jorge AS, Menolli RA. Relationships among antibodies against extractable nuclear antigens, antinuclear antibodies, and autoimmune diseases in a Brazilian public hospital. Autoimmune Dis. 2018;2018:9856910. https://doi.org/10.1155/2018/9856910.
Garcia-Reyero J, Martinez Magunacelaya N, Gonzalez de Villambrosia S, Loghavi S, Gomez Mediavilla A, Tonda R, Beltran S, Gut M, Pereña Gonzalez A, d’Ámore E, Visco C, Khoury JD, Montes-Moreno S. Genetic lesions in MYC and STAT3 drive oncogenic transcription factor overexpression in plasmablastic lymphoma. Haematologica. 2021;106(4):1120–8. https://doi.org/10.3324/haematol.2020.251579.
Damoiseaux JG, Tervaert JW. From ANA to ANA-specific-autoantibodies: how to proceed? Autoimmun Rev. 2006;5(1):10–7. https://doi.org/10.1016/j.autrev.2005.05.007.
González DA, Rodríguez CC, Armas LM, Varela AR, Rodríguez IM, Duarte MT, de León AC. ANA-specific antibodiesprofiles related with anti-SS-A/Ro. The detection of Ro52 and Ro60 according to the presence of SS-B/La, and ANA pattern and titer. Immunol Lett. 2014;161(1):6–12. https://doi.org/10.1016/j.imlet.2014.04.009.
Kanani FH, Hussain T, Talat T, Ghouri N. Frequencies and results of anti-nuclear, anti-dsDNA, and ANA-specific antibodiesin a tertiary-care hospital in Karachi. Pakistan J Pak Med Assoc. 2021;71(2B):708–12. https://doi.org/10.47391/JPMA.1301.
Tipu HN, Bashir MM. Determination of specificity and pattern of antinuclear antibodies (ANA) in systemic rheumatic disease patients positive for ANA testing. J Coll Phys Surg Pak. 2018;28(1):40–3. https://doi.org/10.29271/jcpsp.2018.01.40.
Tozzoli R, D’Aurizio F, Villalta D, Bizzaro N. Automation, consolidation, and integration in autoimmune diagnostics. Auto Immun Highlights. 2015;6(1–2):1–6. https://doi.org/10.1007/s13317-015-0067-5.
Van Hoovels L, Broeders S, Chan EKL, Andrade L, de Melo CW, Damoiseaux J, Viander M, Herold M, Coucke W, Heijnen I, Bogdanos D, Calvo-Alén J, Eriksson C, Kozmar A, Kuhi L, Bonroy C, Lauwerys B, Schouwers S, Lutteri L, Vercammen M, Mayer M, Patel D, Egner W, Puolakka K, Tesija-Kuna A, Shoenfeld Y, de Sousa MJR, Hoyos ML, Radice A, Bossuyt X. Current laboratory and clinical practices in reporting and interpreting anti-nuclear antibody indirect immunofluorescence (ANA IIF) patterns: results of an international survey. Auto Immun Highlights. 2020;11(1):17. https://doi.org/10.1186/s13317-020-00139-9.
Infantino M, Carbone T, Manfredi M, Grossi V, Antico A, Panozzo MP, Brusca I, Alessio MG, Previtali G, Platzgummer S, Cinquanta L, Paura G, Deleonardi G, Trevisan MT, Radice A, Castiglione C, Imbastaro T, Fabris M, Pesce G, Porcelli B, Terzuoli L, Sorrentino MC, Tampoia M, Abbracciavento L, Villalta D, Conte M, Barberio G, Gallo N, Benucci M, Bizzaro N. Study Group on Autoimmune Diseases of the Italian Society of Clinical Pathology and Laboratory Medicine. A new diagnostic algorithm for pattern-oriented autoantibody testing according to the ICAP nomenclature: a pilot study. Autoimmun Rev. 2020;19(8):102588. https://doi.org/10.1016/j.autrev.2020.102588.
Lyons R, Narain S, Nichols C, Satoh M, Reeves WH. Effective use of autoantibody tests in the diagnosis of systemic autoimmune disease. Ann N Y Acad Sci. 2005;1050:217–28. https://doi.org/10.1196/annals.1313.023.
Tešija Kuna A, Đerek L, Drvar V, Kozmar A, Gugo K. Assessment of antinuclear antibodies (ANA): national recommendations on behalf of the Croatian society of medical biochemistry and laboratory medicine. Biochem Med (Zagreb). 2021;31(2):020502. https://doi.org/10.11613/BM.2021.020502.
Alsubki R, Tabassum H, Alfawaz H, Alaqil R, Aljaser F, Ansar S, Al JA. Association between antinuclear antibodies (ANA) patterns and extractable nuclear antigens (ANA-specific-autoantibodies) in HEp-2 cells in patients with autoimmune diseases in Riyadh. Saudi Arabia Intractable Rare Dis Res. 2020;9(2):89–94. https://doi.org/10.5582/irdr.2020.03012.
Rodríguez-Orozco AR, Béjar-Lozano C, Cortés-Rojo C. Antibodies to extractable nuclear antigens are detectable in a considerable number of sera that test negative for antinuclear antibodies. Arch Pathol Lab Med. 2022;146(2):143–4. https://doi.org/10.5858/arpa.2021-0078-LE.
Sarfaraz S, Anis S, Ahmed E, Muzaffar R. Clinical significance of anti-ribosomal P protein antibodies in patients with lupus nephritis. Rev Recent Clin Trials. 2018;13(4):281–6. https://doi.org/10.2174/1574887113666180409154641.
Chan EKL, von Mühlen CA, Fritzler MJ, Damoiseaux J, Infantino M, Klotz W, Satoh M, Musset L. García-De La Torre I, Carballo OG, Herold M, de Melo Cruvinel W, Mimori T, Andrade LEC; ICAP Committee. The International Consensus on ANA Patterns (ICAP) in 2021-the 6th workshop and current perspectives. J Appl Lab Med. 2022;7(1):322–30. https://doi.org/10.1093/jalm/jfab140.
Baek HM, Nahm CH, Song JS, Park W, Moon YS, Kim JJ. Clinical significance of cytoplasmic staining in antinuclear antibody tests using HEp-2 cells. Korean J Lab Med. 2003;23(6):415–9.
Yang Z, Ren Y, Liu D, Lin F, Liang Y. Prevalence of systemic autoimmune rheumatic diseases and clinical significance of ANA profile: data from a tertiary hospital in Shanghai. China APMIS. 2016;124(9):805–11. https://doi.org/10.1111/apm.12564.
Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–79. https://doi.org/10.1038/s41584-020-0480-7.
Dema B, Charles N. Autoantibodies in SLE: specificities, isotypes and receptors. Antibodies (Basel). 2016;5(1):2. https://doi.org/10.3390/antib5010002.
Didier K, Bolko L, Giusti D, Toquet S, Robbins A, Antonicelli F, Servettaz A. Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol. 2018;9:541. https://doi.org/10.3389/fimmu.2018.00541.
Damoiseaux J, von Mühlen CA, Garcia-De La Torre I, Carballo OG, de Melo Cruvinel W, Francescantonio PL, Fritzler MJ, Herold M, Mimori T, Satoh M, Andrade LE, Chan EK, Conrad K. International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Auto Immun Highlights. 2016;7(1):1.
Rekvig OP. Autoimmunity and SLE: factual and semantic evidence-based critical analyses of definitions, etiology, and pathogenesis. Front Immunol. 2020;11: 569234. https://doi.org/10.3389/fimmu.2020.569234.
Jury EC, D’Cruz D, Morrow WJ. Autoantibodies and overlap syndromes in autoimmune rheumatic disease. J Clin Pathol. 2001;54(5):340–7. https://doi.org/10.1136/jcp.54.5.340.
Acknowledgements
We are extremely grateful to Mr. Jaffer Bin Baqar (Sr. Manager Research, Getz Pharma) for his assistance with the statistical analysis and data presentations. We acknowledge the efforts of Ms. Mamona Mushtaq to help us with extracting and compiling data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr. Sabiha Anis. The first and final draft of the manuscript was written by Dr. Sabiha Anis and all authors reviewed and commented on the manuscript for final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anis, S., Fatima, A., Abdul Jabbar, S. et al. ANA-specific antibodies, ANA patterns, anti-ds-DNA results, and clinical diagnosis: a laboratory and clinical audit. Immunol Res 71, 267–275 (2023). https://doi.org/10.1007/s12026-022-09347-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09347-z