Abstract
Purpose
To study the association between testosterone and non-alcoholic fatty liver disease (NAFLD) since prior studies have reported inconsistent results.
Methods
A retrospective analysis was performed including obese men who underwent a liver biopsy and a metabolic and hepatological work-up. Free testosterone (CFT) was calculated by the Vermeulen equation. The association between total testosterone (total T) and CFT on the one hand and NAFLD and fibrosis on the other hand was investigated and corrected for biasing factors such as metabolic parameters.
Results
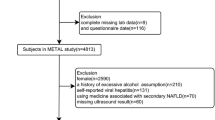
In total, 134 men (mean age 45 ± 12 years, median BMI 39.6 (25.0–64.9) kg/m²) were included. The level of total T and CFT did not significantly differ between NAFL and NASH and the stages of steatosis and ballooning. CFT was significantly lower in a higher stage of fibrosis (p = 0.013), not seen for total T and not persisting after controlling for the influence of BMI, HDL cholesterol and HOMA-IR. A higher stage of lobular inflammation was associated with a lower level of total T (p = 0.033), not seen for CFT and not persisting after controlling for the influence of visceral adipose tissue surface and HOMA-IR.
Conclusions
This is the second largest study investigating the association between testosterone and biopsy-proven NAFLD. No significant association between testosterone levels and NAFLD, and the different histological subgroups or fibrosis was seen. The lower level of CFT in a higher stage of fibrosis and the association between total T and lobular inflammation was driven by poor metabolic parameters.

Similar content being viewed by others
Data availability
All datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- NAFLD:
-
non-alcoholic fatty liver disease
- NAFL:
-
non-alcoholic fatty liver
- NASH:
-
non-alcoholic steatohepatitis
- VAT:
-
visceral adipose tissue surface on CT scan
- SHBG:
-
sex hormone binding globulin
- OE:
-
oestradiol
- total T:
-
total testosterone
- SAT:
-
subcutaneous adipose tissue surface on CT scan
- AHT:
-
arterial hypertension
- HOMA-IR:
-
homeostatic model assessment of insulin resistance
- LH:
-
luteinizing hormone
- FSH:
-
follicle stimulating hormone
- CFT:
-
calculated free testosterone
- FT:
-
free testosterone
- BMI:
-
body mass index
- HDL:
-
high-density lipoprotein
References
P. Bedossa, Pathology of non-alcoholic fatty liver disease. Liver Int. 37(Suppl 1), 85–89 (2017)
M. Blachier, H. Leleu, M. Peck-Radosavljevic, D.C. Valla, F. Roudot-Thoraval, The burden of liver disease in Europe: a review of available epidemiological data. J. Hepatol. 58(3), 593–608 (2013)
J.T. Haas, S. Francque, B. Staels, Pathophysiology and mechanisms of nonalcoholic Fatty Liver Disease. Annu Rev. Physiol. 78, 181–205 (2016)
N. Chalasani, Z. Younossi, J.E. Lavine, M. Charlton, K. Cusi, M. Rinella et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1), 328–57. (2018)
H. Hagstrom, P. Nasr, M. Ekstedt, U. Hammar, P. Stal, R. Hultcrantz et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67(6), 1265–73. (2017)
E. Fabbrini, S. Sullivan, S. Klein, Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51(2), 679–689 (2010)
M.N. Fui, P. Dupuis, M. Grossmann, Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J. Androl. 16(2), 223–231 (2014)
D.M. Kelly, T.H. Jones, Testosterone and obesity. Obes. Rev. 16(7), 581–606 (2015)
M. Grossmann, Hypogonadism and male obesity: focus on unresolved questions. Clin. Endocrinol. (Oxf.) 89(1), 11–21 (2018)
P. Dandona, S. Dhindsa, Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab. 96(9), 2643–2651 (2011)
T. Mushannen, P. Cortez, F.C. Stanford, V. Singhal, Obesity and hypogonadism—a narrative review highlighting the need for high-quality data in adolescents. Children (Basel). 6(5) (2019)
Sumida. The Association of Low Free Testosterone with Histological Severity of Nonalcoholic Fatty Liver Disease in Japanese Men. gastroenterology and hepatology: open acces. 2(4) (2015)
F. Van de Velde, M. Bekaert, A. Hoorens, A. Geerts, G. T’Sjoen, T. Fiers et al. Histologically proven hepatic steatosis associates with lower testosterone levels in men with obesity. Asian J. Androl. 22(3), 252–257 (2020)
K.A. Dayton, F. Bril, D. Barb, J. Lai, S. Kalavalapalli, K. Cusi, Severity of non-alcoholic steatohepatitis is not linked to testosterone concentration in patients with type 2 diabetes. PLoS One 16(6), e0251449 (2021)
M. Sarkar, K. Yates, A. Suzuki, J. Lavine, R. Gill, T. Ziegler et al. Low testosterone is associated with nonalcoholic steatohepatitis and fibrosis severity in men. Clin. Gastroenterol. Hepatol. 19(2), 400–402 (2021)
E. Maseroli, P. Comeglio, C. Corno, I. Cellai, S. Filippi, T. Mello et al. Testosterone treatment is associated with reduced adipose tissue dysfunction and nonalcoholic fatty liver disease in obese hypogonadal men. J. Endocrinol. Invest. 44(4), 819–42. (2021)
K. van der Kooy, J.C. Seidell, Techniques for the measurement of visceral fat: a practical guide. Int. J. Obes. Relat. Metab. Disord. 17(4), 187–196 (1993)
D.R. Matthews, J.P. Hosker, A.S. Rudenski, B.A. Naylor, D.F. Treacher, R.C. Turner, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985)
A. Vermeulen, L. Verdonck, J.M. Kaufman, A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 84(10), 3666–3672 (1999)
J.L. Shea, P.Y. Wong, Y. Chen, Free testosterone: clinical utility and important analytical aspects of measurement. Adv. Clin. Chem. 63, 59–84 (2014)
P. Bedossa, C. Poitou, N. Veyrie, J.L. Bouillot, A. Basdevant, V. Paradis et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56(5), 1751–1759 (2012)
E.P. Stahl, D.S. Dhindsa, S.K. Lee, P.B. Sandesara, N.P. Chalasani, L.S. Sperling, Nonalcoholic Fatty Liver Disease and the Heart: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73(8), 948–63. (2019)
G. Mintziori, P. Poulakos, C. Tsametis, D.G. Goulis, Hypogonadism and non-alcoholic fatty liver disease. Minerva Endocrinol. 42(2), 145–50. (2017)
V. Jaruvongvanich, A. Sanguankeo, T. Riangwiwat, S. Upala, Testosterone, sex hormone-binding globulin and nonalcoholic Fatty Liver Disease: a Systematic review and meta-analysis. Ann. Hepatol. 16(3), 382–94. (2017)
J.G. Fan, S.U. Kim, V.W. Wong, New trends on obesity and NAFLD in Asia. J. Hepatol. 67(4), 862–73. (2017)
G. Bedogni, L. Miglioli, F. Masutti, C. Tiribelli, G. Marchesini, S. Bellentani, Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42(1), 44–52 (2005)
A.L. Fracanzani, S. Petta, R. Lombardi, G. Pisano, M. Russello, D. Consonni et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin. Gastroenterol. Hepatol. 15(10), 1604–1611 (2017)
J. Luo, Q. Chen, T. Shen, X. Wang, W. Fang, X. Wu, et al. Association of sex hormone-binding globulin with nonalcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond). 15, 79 (2018)
C. Lee, J. Kim, Y. Jung, Potential therapeutic application of estrogen in gender disparity of nonalcoholic Fatty Liver Disease/nonalcoholic steatohepatitis. Cells 8(10), (2019)
J.K. DiStefano, NAFLD and NASH in postmenopausal women: implications for diagnosis and treatment. Endocrinology. 161(10), (2020)
X. Zhang, Y. Mou, E. Aribas, M. Amiri, J. Nano, W.M. Bramer, et al. Associations of sex steroids and sex hormone-binding globulin with non-alcoholic Fatty Liver Disease: a population-based study and meta-analysis. Genes (Basel). 13(6), (2022)
Funding
The work has been supported by the HEPADIP Consortium (Hepatic and adipose tissue and functions in the metabolic syndrome) supported by the European Commission as an Integrated Project under the 6th Framework Program (Contract LSHM-CT-2005-018734).
Author information
Authors and Affiliations
Contributions
E.D., C.D.B. and L.V.G. contributed to the design and implementation of the research. A.V. and K.V.D. collected the data. C.D.H. wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics
Every patient underwent a metabolic and hepatological work-up approved by the Ethics Committee of the Antwerp University Hospital (reference 6/25/125, Belgian registration number B30020071389).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Herdt, C., De Block, C., Francque, S. et al. A cross-sectional analysis of the association between testosterone and biopsy-proven non-alcoholic fatty liver disease in men with obesity. Endocrine 80, 54–63 (2023). https://doi.org/10.1007/s12020-022-03245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03245-y