Abstract
Bile acids are signalling hormones involved in the regulation of several metabolic pathways. The ability of bile acids to bind and signal through their receptors is modulated by the gut microbiome, since the microbiome contributes to the regulation and synthesis of bile acids as well to their physiochemical properties. From the gut, bacteria have been shown to send signals to the central nervous system via their metabolites, thus affecting the behaviour and brain function of the host organism. In the last years it has become increasingly evident that bile acids affect brain function, during normal physiological and pathological conditions. Although bile acids may be synthesized locally in the brain, the majority of brain bile acids are taken up from the systemic circulation. Since the composition of the brain bile acid pool may be regulated by the action of intestinal bacteria, it is possible that bile acids function as a communication bridge between the gut microbiome and the brain. However, little is known about the molecular mechanisms and the physiological roles of bile acids in the central nervous system. The possibility that bile acids may be a direct link between the intestinal microbiome and the brain is also an understudied subject. Here we review the influence of gut bacteria on the bile acid pool composition and properties, as well as striking evidence showing the role of bile acids as neuroactive molecules.


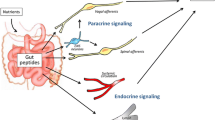
Source of bile acids in the brain. Approximately 95% of the bile acids released into the intestine and transformed by the gut bacteria are re-absorbed at the brush border of the enterocytes. Bile acids are then transport via the portal circulation and taken up into hepatocytes by the basolateral bile acid transporters. A small amount of the bile acids in the enterohepatic circulation escape to the systemic circulation from where they can be taken up into the brain by passive diffusion or by active transport through the blood brain barrier (BBB). Additionally, the primary bile acids CA and CDCA can also be synthesized in the brain via the alternative pathway (involving the BA synthesis enzymes CYP27A1, CYP7B1 and CYP8B1) or via CYP46A1. Although the hepatic bile acid transporters are found in the BBB and in the brain, if they transport bile acids in the brain, and the directionality of their bile acid transport are unknown. CYP7A1 cholesterol 7α-hydroxylase, CYP8B1 12α-hydroxylase, CYP27A1 27-hydroxylase, CYP7B1 sterol 7α-hydroxylase, CYP46A1 cholesterol 24-hydroxylase, CA cholic acid, CDCA chenodeoxycholic acid, BBB blood brain barrier
Similar content being viewed by others
References
Adada, M., Canals, D., Hannun, Y. A., & Obeid, L. M. (2013). Sphingosine-1-phosphate receptor 2. FEBS Journal, 280(24), 6354–6366.
Akahoshi, N., Ishizaki, Y., Yasuda, H., Murashima, Y. L., Shinba, T., Goto, K., et al. (2011). Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking Sphingosine 1-phosphate receptor 2. Epilepsy & Behavior, 22(4), 659–665.
Amador, M. M., Masingue, M., Debs, R., Lamari, F., Perlbarg, V., Roze, E., et al. (2018). Treatment with chenodeoxycholic acid in Cerebrotendinous Xanthomatosis: Clinical, neurophysiological, and quantitative brain structural outcomes. Journal of Inherited Metabolic Disease, 41(5), 799–807.
Angelin, B., Björkhem, I., Einarsson, K., & Ewerth, S. (1982). Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. The Journal of Clinical Investigation, 70(4), 724–731.
Bates, G. P., Dorsey, R., Gusella, J. F., Hayden, M. R., Kay, C., Leavitt, B. R., et al. (2015). Huntington disease. Nature Reviews Disease Primers, 1(1), 15005.
Bazzari, F. H., Abdallah, D. M., & El-Abhar, H. S. (2019). Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules, 24(10), 192.
Begley, M., Hill, C., & Gahan, C. G. M. (2006). Bile Salt Hydrolase activity in probiotics. Applied and Environmental Microbiology, 72(3), 1729–1738.
Bengmark, S. (2013). Gut microbiota, immune development and function. Pharmacological Research, 69(1), 87–113.
Bian, K. Y., Jin, H. F., Sun, W., & Sun, Y. J. (2019). DCA can improve the ACI-induced neurological impairment through negative regulation of Nrf2 signaling pathway. European Review for Medical and Pharmacological Sciences, 23(1), 343–351.
Boussicault, L., Alves, S., Lamazière, A., Planques, A., Heck, N., Moumné, L., et al. (2016). CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain, 139(Pt 3), 953–970.
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences, 108(38), 16050–16055.
Cali, J. J., Hsieh, C. L., Francke, U., & Russell, D. W. (1991). Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie Cerebrotendinous Xanthomatosis. Journal of Biological Chemistry, 266(12), 7778–7783.
Carvajal, F. J., Mattison, H. A., & Cerpa, W. (2016). Role of NMDA receptor-mediated glutamatergic signaling in chronic and acute neuropathologies. Neural Plasticity, 2016, 2701526.
Castro-Caldas, M., Carvalho, A. N., Rodrigues, E., Henderson, C. J., Wolf, C. R., Rodrigues, C. M. P., et al. (2012). Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Molecular Neurobiology, 46(2), 475–486.
Chandler, C. E., Ernst, R. K. (2017). Bacterial lipids: Powerful modifiers of the innate immune response. F1000Research 6.
Chen, Z., Jalabi, W., Shpargel, K. B., Farabaugh, K. T., Dutta, R., Yin, X., et al. (2012). Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. The Journal of Neuroscience, 32(34), 11706–11715.
Chiang, J. Y. L., & Ferrell, J. M. (2019). Bile acids as metabolic regulators and nutrient sensors. Annual Review of Nutrition, 39, 175–200.
Choudhuri, S., Cherrington, N. J., Li, N., & Klaassen, C. D. (2003). Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metabolism and Disposition, 31(11), 13371345.
Collins, S. M., Surette, M., & Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews. Microbiology, 10(11), 735–742.
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W., & Kelley, K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience, 9(1), 46–56.
Dawson, P. A. (2010). Bile secretion and the enterohepatic circulation. Gastrointetinal and Liver Disease, 1, 1075–1088.
De Aguiar Vallim, T. Q., Tarling, E. J., & Edwards, P. A. (2013). Pleiotropic roles of bile acids in metabolism. Cell Metabolism, 17(5), 657–669.
De Giorgio, F., Maduro, C., Fisher, E.M.C., Acevedo-Arozena, A. (2019). Transgenic and physiological mouse models give insights into different aspects of Amyotrophic Lateral Sclerosis. Disease Models and Mechanisms 12(1):dmm037424.
Dinan, T. G., Stilling, R. M., Stanton, C., & Cryan, J. F. (2015). Collective unconscious: How gut microbes shape human behavior. Journal of Psychiatric Research, 63, 1–9.
Ding, L., Yang, L., Wang, Z., & Huang, W. (2015). Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharmaceutica Sinica B, 5(2), 135–144.
Dionísio, P. A., Amaral, J. D., Ribeiro, M. F., Lo, A. C., D’Hooge, R., & Rodrigues, C. M. P. (2015). Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiology of Aging, 36(1), 228–240.
Donnan, G. A., Fisher, M., Macleod, M., & Davis, S. M. (2008). Stroke. Lancet, 371(9624), 1612–1623.
El Aidy, S., Dinan, T. G., & Cryan, J. F. (2014). Immune modulation of the brain-gut-microbe axis. Frontiers in Microbiology, 5, 146.
Elia, A. E., Lalli, S., Monsurrò, M. R., Sagnelli, A., Taiello, A. C., Reggiori, B., et al. (2016). Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. European Journal of Neurology, 23(1), 45–52.
Erny, D., de Angelis, A. L. H., Jaitin, D., Wieghofer, P., Staszewski, O., & David, E. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18(7), 965–977.
Eyles, D. W., Smith, S., Kinobe, R., Hewison, M., & McGrath, J. J. (2005). Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. Journal of Chemical Neuroanatomy, 29(1), 21–30.
Fiorucci, S., Mencarelli, A., Palladino, G., & Cipriani, S. (2009). Bile-acid-activated receptors: Targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends in Pharmacological Sciences, 30(11), 570–580.
Fuchikami, M., Yamamoto, S., Morinobu, S., Okada, S., Yamawaki, Y., & Yamawaki, S. (2016). The potential use of histone deacetylase inhibitors in the treatment of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 64, 320–324.
Fülling, C., Dinan, T. G., & Cryan, J. F. (2019). Gut microbe to brain signaling: What happens in vagus…. Neuron, 101(6), 998–1002.
Fung, T. C., Vuong, H. E., Luna, C. D. G., Pronovost, G. N., Aleksandrova, A. A., Riley, N. G., et al. (2019). Intestinal serotonin and fluoxitine exposure modulate bacterial colonization in the gut. Nature Microbiology, 4, 2064–2073.
Graham, S. F., Rey, N. L., Ugur, Z., Yilmaz, A., Sherman, E., Maddens, M., et al. (2018). Metabolomic profiling of bile acids in an experimental model of prodromal Parkinson’s disease. Metabolites, 8(4), 71.
Grenham, S., Clarke, G., Cryan, J. F., & Dinan, T. G. (2011). Brain-gut-microbe communication in health and disease. Frontiers in Physiology, 2, 94.
Haberland, M., Montgomery, R. L., & Olson, E. N. (2009). The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nature Reviews. Genetics, 10(1), 32–42.
Han, S., Li, T., Ellis, E., Strom, S., & Chiang, J. Y. L. (2010). A novel bile acid-activated Vitamin D receptor signaling in human hepatocytes. Molecular Endocrinology, 24(6), 1151–1164.
Harrison, F., Roberts, A. E. L., Gabrilska, R., Rumbaugh, K. P., Lee, C., & Diggle, S. P. (2015). A 1,000-year-old antimicrobial remedy with antistaphylococcal activity. MBio, 6(4), e01129.
Harrison, I. F., & Dexter, D. T. (2013). Epigenetic targeting of histone deacetylase: Therapeutic potential in Parkinson’s Disease? Pharmacology & Therapeutics, 140(1), 34–52.
Hartmann, P., Hochrath, K., Horvath, A., Chen, P., Seebauer, C. T., & Llorente, C. (2018). Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology, 67(6), 2150–2166.
Hasuike, Y., Endo, T., Koroyasu, M., Matsui, M., Mori, C., Yamadera, M., et al. (2020). Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson’s disease. Medical Hypotheses, 134, 109436.
Hata, T., Asano, Y., Yoshihara, K., Kimura-Todani, T., Miyata, N., Zhang, X.-T., et al. (2017). Regulation of gut lumial serotonin by commensal microbiota in mice. PLoS ONE, 12(7), e0180745.
Heubi, J. E., Setchell, K. D. R., & Bove, K. E. (2007). Inborn errors of bile acid metabolism. Seminars in Liver Disease, 27(3), 282–294.
Higashi, T., Watanabe, S., Tomaru, K., Yamazaki, W., Yoshizawa, K., Ogawa, S., et al. (2017). Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids, 125, 107–113.
Hilton, D., Stephens, M., Kirk, L., Edwards, P., Potter, R., Zajicek, J., et al. (2014). Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathologica, 127(2), 235–241.
Hölscher, C. (2020). Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opinion on Investigational Drugs, 29(4), 333–348.
Huang, C., Wang, J., Hu, W., Wang, C., Lu, X., Tong, L., et al. (2016). Identification of functional farnesoid X receptors in brain neurons. FEBS Letters, 590(18), 3233–3242.
Huang, F., Wang, T., Lan, Y., Yang, L., Pan, W., Zhu, Y., et al. (2015). Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Frontiers in Behavioral Neuroscience, 9, 70.
Inagaki, T., Moschetta, A., Lee, Y. K., Peng, Li., Zhao, G., Downes, M., et al. (2006). Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3920–3925.
Iusuf, D., De Steeg, E. V., & Schinkel, A. H. (2012). Functions of OATP1A and 1B transporters in vivo: Insights from mouse models. Trends in Pharmacological Sciences, 33(2), 100–108.
Joyce, S. A., MacSharry, J., Casey, P. G., Kinsella, M., Murphy, E. F., Shanahan, F., et al. (2014). Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proceedings of the National Academy of Sciences of the United States of America, 11(20), 7421–7426.
Kaemmerer, W. F., Rodrigues, C. M., Steer, C. J., & Low, W. C. (2001). Creatine-supplemented diet extends Purkinje cell survival in Spinocerebellar Ataxia Type 1 transgenic mice but does not prevent the ataxic phenotype. Neuroscience, 103(3), 713–724.
Karran, E., Mercken, M., & De Strooper, B. (2011). The Amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nature Reviews Drug Discovery, 10(9), 698–712.
Kawamata, Y., Fujii, R., Hosoya, M., Harada, M., Yoshida, H., Miwa, M., et al. (2003). A G protein-coupled receptor responsive to bile acids. Journal of Biological Chemistry, 278(11), 9435–9440.
Keene, C. D., Rodrigues, C. M. P., Eich, T., Chhabra, M. S., Steer, C. J., & Low, W. C. (2002). Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proceedings of the National Academy of Sciences, 99(16), 10671–10676.
Keitel, V., Donner, M., Winandy, S., Kubitz, R., & Haussinger, D. (2008). Expression and function of the bile acid receptor TGR5 in kupffer cells. Biochemical and Biophysical Research Communications, 372(1), 78–84.
Keitel, V., Görg, B., Bidmon, H. J., Zemtsova, I., Spomer, L., Zilles, K., et al. (2010). The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. GLIA, 58(15), 1794–1805.
Kempf, A., Tews, B., Arzt, M. E., Weinmann, O., Obermair, F. J., Pernet, V., et al. (2014). The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biology, 12(1), e1001763.
Kim, G. S., Yang, L., Zhang, G., Zhao, H., Selim, M., McCullough, L. D., et al. (2015). Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nature Communications, 6, 7893.
Kim, K.-S., Seeley, R. J., & Sandoval, D. A. (2018). Signalling from the periphery to the brain that regulates energy homeostasis. Nature Reviews. Neuroscience, 19(4), 185–196.
Koch, A., Bonus, M., Gohlke, H., & Klocker, N. (2019). Isoform-specific inhibition of N-methyl-D-aspartate receptors by bile salts. Scientific Reports, 9(1), 10068.
Kotti, T. J., Ramirez, D. M. O., Pfeiffer, B. E., Huber, K. M., & Russell, D. W. (2006). Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3869–3874.
Kurdi, P., Kawanishi, K., Mizutani, K., & Yokota, A. (2006). Mechanism of growth inhibition by free bile acids in Lactobacilli and Bifidobacteria. Journal of Bacteriology, 188(5), 1979–1986.
Labbadia, J., & Morimoto, R. I. (2013). Huntington’s disease: Underlying molecular mechanisms and emerging concepts. Trends in Biochemical Sciences, 38(8), 378–385.
Lamba, V., Yasuda, K., Lamba, J. K., Assem, M., Davila, J., Strom, S., et al. (2004). PXR (NR1I2): Splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicology and Applied Pharmacology, 199(3), 251–265.
Lawson, M. A., Parrott, J. M., McCusker, R. H., Dantzer, R., Kelley, K. W., & O’Connor, J. C. (2013). Intracerebroventricular administration of lipopolysaccharide induces Indoleamine-2,3-dioxygenase-dependent depression-like behaviors. Journal of Neuroinflammation, 10, 87.
Li, L., Liu, C., Mao, W., Tumen, B., & Li, P. (2019). Taurochenodeoxycholic acid inhibited AP-1 activation via stimulating glucocorticoid receptor. Molecules, 24(24), 4513.
Liu, S., Marcelin, G., Blouet, C., Jeong, J. H., Jo, Y.-H., Schwartz, G. J., et al. (2018). A gut-brain axis regulating glucose metabolism mediated by bile acids and competitive fibroblast growth factor actions at the hypothalamus. Molecular Metabolism, 8, 37–50.
Lo, A. C., Callaerts-Vegh, Z., Nunes, A. F., Rodrigues, C. M. P., & D’Hooge, R. (2013). Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiology of Disease, 50, 21–29.
Lorbek, G., Lewinska, M., & Rozman, D. (2012). Cytochrome P450s in the synthesis of cholesterol and bile acids: From mouse models to human diseases. FEBS Journal, 279(9), 1516–1533.
Lorenzo-Zúñiga, V., Bartolí, R., Planas, R., Hofmann, A. F., Viñado, B., Hagey, L. R., et al. (2003). Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology, 37(3), 551–557.
MacLennan, A. J., Carney, P. R., Zhu, W. J., Chaves, A. H., Garcia, J., Grimes, J. R., et al. (2001). An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. The European Journal of Neuroscience, 14(2), 203–209.
Mahgoub, M., & Monteggia, L. M. (2013). Epigenetics and psychiatry. Neurotherapeutics, 10(4), 734–741.
MahmoudianDehkordi, S., Arnold, M., Nho, K., Ahmad, S., Jia, W., Xie, G., et al. (2019). Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimer’s & Dementia, 15(1), 76–92.
Mancuso, R., & Navarro, X. (2015). Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Progress in Neurobiology, 133, 1–126.
Mandia, D., Chaussenot, A., Besson, G., Lamari, I. F., Castelnovo, G., Curot, J., et al. (2019). Cholic acid as a treatment for cerebrotendinous xanthomatosis in adults. Journal of Neurology, 266(8), 2043–2050.
Mano, N., Goto, T., Uchida, M., Nishimura, K., Ando, M., Kobayashi, N., et al. (2004). Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. Journal of Lipid Research, 45(2), 295–300.
Mano, N., Sato, Y., Nagata, M., Goto, T., & Goto, J. (2004). Bioconversion of 3beta-hydroxy-5-cholenoic acid into chenodeoxycholic acid by rat brain enzyme systems. Journal of Lipid Research, 45(9), 1741–1748.
Maruyama, T., Miyamoto, Y., Nakamura, T., Tamai, Y., Okada, H., Sugiyama, E., et al. (2002). Identification of membrane-type receptor for bile acids (M-BAR). Biochemical and Biophysical Research Communications, 298(5), 714–719.
McMillin, M., Frampton, G., Grant, S., Khan, S., Diocares, J., Petrescu, A., et al. (2017). Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Frontiers in Cellular Neuroscience, 11, 191.
McMillin, M., Frampton, G., Quinn, M., Ashfaq, S., De Los Santos, M., Grant, S., et al. (2016). Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. American Journal of Pathology, 186(2), 312–323.
McMillin, M., Frampton, G., Quinn, M., Divan, A., Grant, S., Patel, N., et al. (2015). Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Molecular Endocrinology, 29(12), 1720–1730.
McMillin, M., Frampton, G., Tobin, R., Dusio, G., Smith, J., Shin, H., et al. (2015). TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. Journal of Neurochemistry, 135(3), 565–576.
Merritt, M. E., & Donaldson, J. R. (2009). Effect of bile salts on the DNA and membrane integrity of enteric bacteria. Journal of Medical Microbiology, 58(pt12), 1533–1541.
Mertens, K. L., Kalsbeek, A., Soeters, M. R., & Eggink, H. M. (2017). Bile acid signalling pathways from the enterohepatic circulation to the central nervous system. Froniers in Neuroscience, 11, 617.
Milnerwood, A. J., & Raymond, L. A. (2010). Early synaptic pathophysiology in neurodegeneration: Insights from Huntington’s disease. Trends in Neurosciences, 33(11), 513–523.
Min, J.-H., Hong, Y.-H., Sung, J.-J., Kim, S.-M., Lee, J. B., & Lee, K.-W. (2012). Oral solubilized ursodeoxycholic acid therapy in amyotrophic lateral sclerosis: A randomized cross-over trial. Journal of Korean Medical Science, 27(2), 200–206.
Molinero, N., Ruiz, L., Sánchez, B., Margolles, A., & Delgado, S. (2019). Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Frontiers in Physiology, 10, 185.
Monte, M. J., Marin, J. J. G., Antelo, A., & Vazquez-Tato, J. (2009). Bile acids: Chemistry, physiology, and pathophysiology. World Journal of Gastroenterology, 15(7), 804–816.
Monteiro-Cardoso, V. F., Oliveira, M. M., Melo, T., Domingues, M. R. M., Moreira, P. I., Ferreiro, E., et al. (2014). Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. Journal of Alzheimer’s Disease, 43(4), 1375–1392.
Mortiboys, H., Aasly, J., & Bandmann, O. (2013). Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain, 136(Pt 10), 3038–3050.
Naqvi, S. H., Ramsey, R. B., & Nicholas, H. J. (1970). Detection of lithocholic acid in multiple sclerosis brain tissue. Lipids, 5(6), 578–580.
Nebert, D. W., & Russell, D. W. (2002). Clinical importance of the cytochromes P450. The Lancet, 360(9340), 1155–1162.
Neufeld, K. M., Kang, N., Bienenstock, J., & Foster, J. A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility, 23(3), 255–264.
Nickols, H. H., & Conn, P. J. (2014). Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiology of Disease, 61, 55–71.
Nie, S., Chen, G., Cao, X., & Zhang, Y. (2014). Cerebrotendinous Xanthomatosis: A comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet Journal of Rare Diseases, 9, 179.
Nishimura, M., Yaguti, H., Yoshitsugu, H., Naito, S., & Satoh, T. (2003). Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi, 123(5), 369–375.
Nunes, A. F., Amaral, J. D., Lo, A. C., Fonseca, M. B., Viana, R. J. S., Callaerts-Vegh, Z., et al. (2012). TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Molecular Neurobiology, 45(3), 440–454.
Pan, X., Elliott, C. T., McGuinness, B., Passmore, P., Kehoe, P. G., Hölscher, C., et al. (2017). Metabolomic profiling of bile acids in clinical and experimental samples of Alzheimer’s disease. Metabolites, 17(2), 28.
Parry, G. J., Rodrigues, C. M. P., Aranha, M. M., Hilbert, S. J., Davey, C., Kelkar, P., et al. (2010). Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic acid in patients with amyotrophic lateral sclerosis. Clinical Neuropharmacology, 33(1), 17–21.
Perl, D. P. (2010). Neuropathology of Alzheimer’s disease. Mount Sinai Journal of Medicine, 77(1), 32–42.
Quinn, M., & DeMorrow, S. (2012). Bile in the brain? A role for bile acids in the central nervous system. Journal of Cell Science & Therapy, 3, 7.
Quinn, M., McMillin, M., Galindo, C., Frampton, G., Pae, H. Y., & DeMorrow, S. (2014). Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Digestive and Liver Disease, 46(6), 527–534.
Quinn, R. A., Melnik, A. V., Vrbanac, A., Fu, T., Patras, K. A., Christy, M. P., et al. (2020). Global chemical effects of the microbiome include new bile-acid conjugations. Nature, 579(7797), 123–129.
Radu, B. M., Osculati, A. M. M., Suku, E., Banciu, A., Tsenov, G., Merigo, F., et al. (2017). All muscarinic acetylcholine receptors (M(1)-M(5)) are expressed in murine brain microvascular endothelium. Scientific Reports, 7(1), 5083.
Rajani, V., Sengar, A. S., & Salter, M. W. (2020). Tripartite signalling by NMDA receptors. Molecular Brain, 13(1), 23.
Ramalho, R. M., Nunes, A. F., Dias, R. B., Amaral, J. D., Lo, A. C., D’Hooge, R., et al. (2013). Tauroursodeoxycholic acid suppresses amyloid β-induced synaptic toxicity in vitro and in APP/PS1 mice. Neurobiology of Aging, 34(2), 551–561.
Ramalho, R. M., Viana, R. J. S., Low, W. C., Steer, C. J., & Rodrigues, C. M. P. (2008). Bile acids and apoptosis modulation: An emerging role in experimental Alzheimer’s disease. Trends in Molecular Medicine, 14(2), 54–62.
Raufman, J. P., Chen, Y., Cheng, K., Compadre, C., Compadre, L., & Zimniak, P. (2002). Selective interaction of bile acids with muscarinic receptors: A case of molecular mimicry. European Journal of Pharmacology, 457(2–3), 77–84.
Renton, A. E., Chiò, A., & Traynor, B. J. (2014). State of play in amyotrophic lateral sclerosis genetics. Nature Neuroscience, 17(1), 17–23.
Ridlon, J. M., Kang, D.-J., & Hylemon, P. B. (2006). Bile salt biotransformations by human intestinal bacteria. Journal of Lipid Research, 47(2), 241–259.
Rochellys, D. H., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3047–3052.
Rodrigues, C. M. P., Sola, S., Nan, Z., Castro, R. E., Ribeiro, P. S., Low, W. C., et al. (2003). Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 6087–6092.
Rodrigues, C. M. P., Spellman, S. R., Solá, S., Grande, A. W., Linehan-Stieers, C., Low, W. C., et al. (2002). Neuroprotection by a bile acid in an acute stroke model in the rat. Journal of Cerebral Blood Flow and Metabolism, 22(4), 463–471.
Rodrigues, C. M. P., Stieers, C. L., Keene, C. D., & Ma, X. (2000). Tauroursodeoxycholic acid partially prevents apoptosis induced by 3-nitropropionic acid: Evidence for a mitochondrial pathway independent of the permeability transition. Journal of Neurochemistry, 75(6), 2368–2379.
Rosa, A. I., Fonseca, I., Nunes, M. J., Moreira, S., Rodrigues, E., Carvalho, A. N., et al. (2017). Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Biochimica et Biophysica Acta, 1863(9), 2171–2181.
Russell, D. W. (2003). The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry, 72, 137–174.
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167(6), 1469–1480.
Sampson, T. R., & Mazmanian, S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell Host & Microbe, 17(5), 565–576.
Sánchez, B., Ruiz, L., Gueimonde, M., Ruas-Madiedo, P., & Margolles, A. (2013). Adaptation of bifidobacteria to the gastrointestinal tract and functional consequences. Pharmacological Research, 69(1), 127–136.
Sayin, S. I., Wahlström, A., Felin, J., Jäntti, S., Marschall, H. U., Bamberg, K., et al. (2013). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism, 17(2), 225–235.
Schubring, S. R., Fleischer, W., Lin, J. S., Haas, H. L., & Sergeeva, O. A. (2012). The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABA A receptors. Neuroscience Letters, 506(2), 322–326.
Shulman, J. M., De Jager, P. L., & Feany, M. B. (2011). Parkinson’s disease: Genetics and pathogenesis. Annual Review of Pathology, 6, 193–222.
Sigel, E., & Steinmann, M. E. (2012). Structure, function, and modulation of GABA(A) receptors. The Journal of Biological Chemistry, 287(48), 40224–40231.
Sonne, D. P., van Nierop, F. S., Kulik, W., Soeters, M. R., Vilsbøll, T., & Knop, F. K. (2016). Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with Type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism, 101(8), 3002–3009.
St-Pierre, M. V., Kullak-Ublick, G. A., Hagenbuch, B., & Meier, P. J. (2001). Transport of bile acids in hepatic and non-hepatic tissues. Journal of Experimental Biology, 204, 1673–1686.
Staudinger, J. L., Goodwin, B., Jones, S. A., Hawkins-Brown, D., MacKenzie, K. I., LaTour, A., et al. (2001). The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proceedings of the National Academy of Sciences, 98(6), 3369–3374.
Stilling, R. M., Dinan, T. G., & Cryan, J. F. (2014). Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes, Brain, and Behavior, 13(1), 69–86.
Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Research, 1693(Pt B), 128–133.
Studer, E., Zhou, X., Zhao, R., Wang, Y., Takabe, K., Nagahashi, M., et al. (2012). Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology, 55(1), 267–276.
Takahashi, S., Fukami, T., Masuo, Y., Brocker, C. N., Xie, C., Krausz, K. W., et al. (2016). Cyp2c70 Is responsible for the species difference in bile acid metabolism between mice and humans. Journal of Lipid Research, 57(12), 2130–2137.
Takigawa, T., Miyazaki, H., Kinoshita, M., Kawarabayashi, N., Nishiyama, K., Hatsuse, K., et al. (2013). Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. American Journal of Physiology, 305(6), G427-438.
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., & Macia, L. (2014). The role of short-chain fatty acids in health and disease. Advances in Immunology, 121, 91–119.
Teo, C. R. L., Wang, W., Hai, Y. L., Lee, C. G., & Chong, S. S. (2008). Single-step scalable-throughput molecular screening for Huntington disease. Clinical Chemistry, 54(6), 964–972.
Thomas, C., Gioiello, A., Noriega, L., Strehle, A., Oury, L., Rizzo, G., et al. (2009). TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabolism, 10(3), 167–177.
Vaz, A. R., Cunha, C., Gomes, C., Schmucki, N., Barbosa, M., & Brites, D. (2015). Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Molecular Neurobiology, 51(3), 864–877.
Wahlström, A., Sayin, S. I., Marschall, H. U., & Bäckhed, F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabolism, 24(1), 41–50.
Wang, H., Chen, J., Hollister, K., Sowers, L. C., & Forman, B. M. (1999). Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Molecular Cell, 3(5), 543–553.
Watanabe, M., Houten, S. M., Mataki, C., Christoffolete, M. A., Kim, B. W., Sato, H., et al. (2006). Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature, 439(7075), 484–489.
Weil, A. (1930). The effect of hemolytic toxins on nervous tissue. Archives of Pathology and Laboratory medicine, 9, 828.
Wikoff, W. R., Anfora, A. T., Liu, J., Schultz, P. J., Lesley, S. A., Peters, E. C., et al. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America, 106(10), 3698–3703.
Wu, C., & Sun, D. (2015). GABA receptors in brain development, function, and injury. Metabolic Brain Disease, 30(2), 367–379.
Wu, Q. J., & Tymianski, M. (2018). Targeting NMDA receptors in stroke: New hope in neuroprotection. Molecular Brain, 11(1), 15.
Xie, G., Wang, X., Jiang, R., Zhao, A., Yan, J., Zheng, X., et al. (2018). Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine, 37, 294–306.
Yanguas-Casás, N., Barreda-Manso, M. A., Nieto-Sampedro, M., & Romero-Ramírez, L. (2014). Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. Journal of Neuroinflammation, 11, 50.
Yanguas-Casás, N., Barreda-Manso, M. A., Nieto-Sampedro, M., & Romero-Ramírez, L. (2017). TUDCA: An agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. Journal of Cellular Physiology, 232(8), 2231–2245.
Zheng, X., Chen, T., Zhao, A., Wang, X., Xie, G., Huang, F., et al. (2016). The brain metabolome of male rats across the lifespan. Scientific Reports, 6, 24125.
Funding
VFM-C and MC were funded by the Singapore International Graduate Award (SINGA). RRS was funded by the National University of Singapore and the Agency for Science, Technology and Research, Singapore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monteiro-Cardoso, V.F., Corlianò, M. & Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromol Med 23, 99–117 (2021). https://doi.org/10.1007/s12017-020-08625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08625-z