Abstract
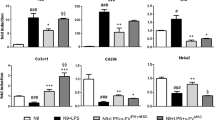
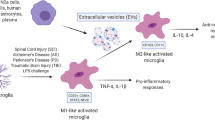
Neurodegenerative diseases (NDDs) continue to be a significant healthcare problem. The economic and social implications of NDDs increase with longevity. NDDs are linked to neuroinflammation and activated microglia and astrocytes play a central role. There is a growing interest for stem cell-based therapy to deliver genes, and for tissue regeneration. The promise of mesenchymal stem cells (MSC) is based on their availability as off-the-shelf source, and ease of expanding from discarded tissues. We tested the hypothesis that MSC have a major role of resetting activated microglial cells. We modeled microglial cell lines by using U937 cell-derived M1 and M2 macrophages. We studied macrophage types, alone, or in a non-contact culture with MSCs. MSCs induced significant release of exosomes from both types of macrophages, but significantly more of the M1 type. RNA sequencing showed enhanced gene expression within the exosomes with the major changes linked to the inflammatory response, including cytokines and the purinergic receptors. Computational analyses of the transcripts supported the expected effect of MSCs in suppressing the inflammatory response of M1 macrophages. The inflammatory cargo of M1 macrophage-derived exosomes revealed involvement of cytokines and purinergic receptors. At the same time, the exosomes from MSC-M2 macrophages were able to reset the classical M2 macrophages to more balanced inflammation. Interestingly, we excluded transfer of purinergic receptor transcripts from the co-cultured MSCs by analyzing these cells for the identified purinergic receptors. Since exosomes are intercellular communicators, these findings provide insights into how MSCs may modulate tissue regeneration and neuroinflammation.
Graphical Abstract








Similar content being viewed by others
References
Hanslik, K. L., Marino, K. M., & Ulland, T. K. (2021). Modulation of glial function in health, aging, and neurodegenerative disease. Frontiers in Cellular Neuroscience, 15, 718324.
Kovacs, G. G. (2016). Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. International Journal of Molecular Sciences, 17, 189.
Gabor, G. (2014). Kovacs current concepts of neurodegenerative diseases. European Medical Journal Neurology, 1, 78–86.
Perry, V. H., & Teeling, J. (2013). Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Seminars in Immunopathology, 35, 601–612.
Ransohoff, R. M., & Perry, V. H. (2009). Microglial physiology: Unique stimuli, specialized responses. Annual Review of Immunology, 27, 119–145.
Sofroniew, M. V., & Vinters, H. V. (2010). Astrocytes: Biology and pathology. Acta neuropathologica, 119, 7–35.
Noh, H., Jeon, J., & Seo, H. (2014). Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochemistry International, 69, 35–40.
Henkel, J. S., Engelhardt, J. I., Siklós, L., Simpson, E. P., Kim, S. H., Pan, T., Goodman, J. C., Siddique, T., Beers, D. R., & Appel, S. H. (2004). Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 55, 221–235.
Mrak, R. E., & Griffin, W. S. T. (2005). Glia and their cytokines in progression of neurodegeneration. Neurobiology of Aging, 26, 349–354.
González, H., Elgueta, D., Montoya, A., & Pacheco, R. (2014). Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. Journal of Neuroimmunology, 274, 1–13.
Gordon, R., Anantharam, V., Kanthasamy, A. G., & Kanthasamy, A. (2012). Proteolytic activation of proapoptotic kinase protein kinase Cδ by tumor necrosis factor α death receptor signaling in dopaminergic neurons during neuroinflammation. Journal of Neuroinflammation, 9, 1–18.
Qian, L., Tan, K. S., Wei, S.-J., Wu, H.-M., Xu, Z., Wilson, B., Lu, R.-B., Hong, J.-S., & Flood, P. M. (2007). Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. The Journal of Immunology, 179, 1198–1209.
Mrinal, K. P., Apala, C., & Soumyabrata, B. (2021). Neurodegeneration: Diagnosis, Prevention, and Therapy. In M. Mahmoud Ahmed (Ed.), Oxidoreductase (p. Ch. 9). Rijeka: IntechOpen.
Sakthiswary, R., & Raymond, A. A. (2012). Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regeneration Research, 7, 1822.
Rameshwar, P., Patel, J., & Aleynik, A. (2017). Stem Cells for Therapeutic Delivery of Mediators and Drugs. In: Toward the Future: The New Challenges of the Cell Therapy and Potential of Regenerative Medicine (pp. 157–157). Bentham Science.
Patel, S. A., Sherman, L., Munoz, J., & Rameshwar, P. (2008). Immunological properties of mesenchymal stem cells and clinical implications. Archivum Immunologiae et Therapiae Experimentalis, 56, 1–8.
Walker, N. D., Elias, M., Guiro, K., Bhatia, R., Greco, S. J., Bryan, M., Gergues, M., Sandiford, O. A., Ponzio, N. M., Leibovich, S. J., & Rameshwar, P. (2019). Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death & Disease, 10, 59.
Sherman, L. S., Munoz, J., Patel, S. A., Dave, M. A., Paige, I., & Rameshwar, P. (2011). Moving from the laboratory bench to patients’ bedside: Considerations for effective therapy with stem cells. Clinical and Translational Science, 4, 380–386.
El-Kheir, W. A., Gabr, H., Awad, M. R., Ghannam, O., Barakat, Y., Farghali, H. A., Maadawi, Z. M. E., Ewes, I., & Sabaawy, H. E. (2014). Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplantation, 23, 729–745.
Akiyama, Y., Radtke, C., & Kocsis, J. D. (2002). Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. Journal of Neuroscience, 22, 6623–6630.
Dezawa, M., Kanno, H., Hoshino, M., Cho, H., Matsumoto, N., Itokazu, Y., Tajima, N., Yamada, H., Sawada, H., & Ishikawa, H. (2004). Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. The Journal of Clinical Investigation, 113, 1701–1710.
Mahmood, A., Lu, D., Wang, L., & Chopp, M. (2002). Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. Journal of Neurotrauma, 19, 1609–1617.
Zhang, J., Li, Y., Chen, J., Cui, Y., Lu, M., Elias, S. B., Mitchell, J. B., Hammill, L., Vanguri, P., & Chopp, M. (2005). Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Experimental Neurology, 195, 16–26.
Sharma, A. (2018). Role of stem cell derived exosomes in tumor biology. International Journal of Cancer, 142, 1086–1092.
Illes, P., Rubini, P., Ulrich, H., Zhao, Y., & Tang, Y. (2020). Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells, 9, 1108.
Wang, J., Takemura, N., & Saitoh, T. (2021). Macrophage response driven by extracellular ATP. Biological and Pharmaceutical Bulletin, 44, 599–604.
Merz, J., Nettesheim, A., von Garlen, S., Albrecht, P., Saller, B., Engelmann, J., Hertle, L., Schäfer, I., Dimanski, D., & König, S. (2021). Pro-and anti-inflammatory macrophages express a sub-type specific purinergic receptor profile. Purinergic Signalling, 17, 481–492.
Grassivaro, F., Menon, R., Acquaviva, M., Ottoboni, L., Ruffini, F., Bergamaschi, A., Muzio, L., Farina, C., & Martino, G. (2020). Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. Journal of Neuroscience, 40, 784–795.
Sinha, G., Ferrer, A. I., Ayer, S., El-Far, M. H., Pamarthi, S. H., Naaldijk, Y., Barak, P., Sandiford, O. A., Bibber, B. M., Yehia, G., Greco, S. J., Jiang, S. G., Bryan, M., Kumar, R., Ponzio, N. M., Etchegaray, J. P., & Rameshwar, P. (2021). Specific N-cadherin-dependent pathways drive human breast cancer dormancy in bone marrow. Life Sci Alliance, 4(7), e202000969.
Bliss, S. A., Sinha, G., Sandiford, O. A., Williams, L. M., Engelberth, D. J., Guiro, K., Isenalumhe, L. L., Greco, S. J., Ayer, S., Bryan, M., Kumar, R., Ponzio, N. M., & Rameshwar, P. (2016). Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Research, 76, 5832–5844.
Sandiford, O. A., Donnelly, R. J., El-Far, M. H., Burgmeyer, L. M., Sinha, G., Pamarthi, S. H., Sherman, L. S., Ferrer, A. I., DeVore, D. E., Patel, S. A., Naaldijk, Y., Alonso, S., Barak, P., Bryan, M., Ponzio, N. M., Narayanan, R., Etchegaray, J. P., Kumar, R., & Rameshwar, P. (2021). Mesenchymal stem cell-secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Research, 81, 1567–1582.
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin-Smith, G. K., Ayre, D. C., Bach, J.-M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., … Zuba-Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750.
Greco, S. J., Ayer, S., Guiro, K., Sinha, G., Donnelly, R. J., Markos, H., Sherman, L. S., Kenfack, Y., Pamarthi, S. H., & Gergues, M. (2021). Restoration of aged hematopoietic cells by their young counterparts through instructive microvesicles release. Aging (Albany NY), 13, 23981.
Eruslanov, E., & Kusmartsev, S. (2010). Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. In D. Armstrong (Ed.), Advanced Protocols in Oxidative Stress II (pp. 57–72). Humana Press.
Dello Russo, C., Cappoli, N., Coletta, I., Mezzogori, D., Paciello, F., Pozzoli, G., Navarra, P., & Battaglia, A. (2018). The human microglial HMC3 cell line: Where do we stand? A systematic literature review. Journal of Neuroinflammation, 15, 259.
Jeong, H. K., Ji, K., Min, K., & Joe, E. H. (2013). Brain inflammation and microglia: Facts and misconceptions. Experimental Neurobiology, 22, 59–67.
Gabrusiewicz, K., Ellert-Miklaszewska, A., Lipko, M., Sielska, M., Frankowska, M., & Kaminska, B. (2011). Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE, 6, e23902.
Janabi, N., Peudenier, S., Héron, B., Ng, K. H., & Tardieu, M. (1995). Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neuroscience Letters, 195, 105–108.
Streit, W. J. (2001). Microglia and macrophages in the developing CNS. Neurotoxicology, 22, 619–624.
Sharp, B. M. (2013). Conversion of the U937 monocyte into “Macrophage-Like” populations exhibiting M1 or M2 characteristics. Master thesis, Wright State University
Bertram, C., von Neuhoff, N., Skawran, B., Steinemann, D., Schlegelberger, B., & Hass, R. (2008). The differentiation/retrodifferentiation program of human U937 leukemia cells is accompanied by changes of VCP/p97. BMC Cell Biology, 9, 1–16.
Fanger, N. A., Wardwell, K., Shen, L., Tedder, T. F., & Guyre, P. M. (1996). Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. The Journal of Immunology, 157, 541–548.
Morrison, T. J., Jackson, M. V., Cunningham, E. K., Kissenpfennig, A., McAuley, D. F., O’Kane, C. M., & Krasnodembskaya, A. D. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. American Journal of Respiratory and Critical Care Medicine, 196, 1275–1286.
Lo Sicco, C., Reverberi, D., Balbi, C., Ulivi, V., Principi, E., Pascucci, L., Becherini, P., Bosco, M. C., Varesio, L., Franzin, C., Pozzobon, M., Cancedda, R., & Tasso, R. (2017). Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Translational Medicine, 6, 1018–1028.
Cekic, C., & Linden, J. (2016). Purinergic regulation of the immune system. Nature Reviews Immunology, 16, 177–192.
Dosch, M., Gerber, J., Jebbawi, F., & Beldi, G. (2018). Mechanisms of ATP release by inflammatory cells. International Journal of Molecular Sciences, 19, 1222.
Arslan, F., Lai, R. C., Smeets, M. B., Akeroyd, L., Choo, A., Aguor, E. N., Timmers, L., van Rijen, H. V., Doevendans, P. A., & Pasterkamp, G. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10, 301–312.
Drago, F., Lombardi, M., Prada, I., Gabrielli, M., Joshi, P., Cojoc, D., Franck, J., Fournier, I., Vizioli, J., & Verderio, C. (2017). ATP modifies the proteome of extracellular vesicles released by microglia and influences their action on astrocytes. Frontiers in Pharmacology, 8, 910.
Lister, M. F., Sharkey, J., Sawatzky, D. A., Hodgkiss, J. P., Davidson, D. J., Rossi, A. G., & Finlayson, K. (2007). The role of the purinergic P2X 7 receptor in inflammation. Journal of Inflammation, 4, 1–14.
Cohen, S., Harpaz, Z., Farbstein, M., Fishman, S., Barer, F., & Fishman, P. (2015). THU0064 the A3 Adenosine Receptor (A3AR): Therapeutic target and predictive biological marker in rheumatoid arthritis. BMJ Publishing Group Ltd.
Erb, L., & Weisman, G. A. (2012). Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling, 1, 789–803.
Aydin, D., Weyer, S. W., & Müller, U. C. (2012). Functions of the APP gene family in the nervous system: Insights from mouse models. Experimental Brain Research, 217, 423–434.
Volkman, R., & Offen, D. (2017). Concise review: Mesenchymal stem cells in neurodegenerative diseases. Stem Cells, 35, 1867–1880.
Aleynik, A., Gernavage, K. M., Mourad, Y., Sherman, L. S., Liu, K., Gubenko, Y. A., & Rameshwar, P. (2014). Stem cell delivery of therapies for brain disorders. Clinical and Translational Medicine, 3, 24.
Kwon, S., Yoo, K. H., Sym, S. J., & Khang, D. (2019). Mesenchymal stem cell therapy assisted by nanotechnology: A possible combinational treatment for brain tumor and central nerve regeneration. International Journal of Nanomedicine, 14, 5925–5942.
de la Rosa, G., Gómez, A. I., Baños, M. C., & Pelegrín, P. (2020). Signaling through purinergic receptor p2y2 enhances macrophage il-1β production. International Journal of Molecular Sciences, 21, 4686.
Klaver, D., & Thurnher, M. (2021). Control of macrophage inflammation by P2Y purinergic receptors. Cells, 10, 1098.
Kreckler, L. M., Wan, T. C., Ge, Z.-D., & Auchampach, J. A. (2006). Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. Journal of Pharmacology and Experimental Therapeutics, 317, 172–180.
Csóka, B., Németh, Z. H., Virág, L., Gergely, P., Leibovich, S. J., Pacher, P., Sun, C.-X., Blackburn, M. R., Vizi, E. S., & Deitch, E. A. (2007). A2A adenosine receptors and C/EBPβ are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood, the Journal of the American Society of Hematology, 110, 2685–2695.
Németh, Z. H., Lutz, C. S., Csóka, B., Deitch, E. A., Leibovich, S. J., Gause, W. C., Tone, M., Pacher, P., Vizi, E. S., & Haskó, Gr. (2005). Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. The Journal of Immunology, 175, 8260–8270.
Niemi, K., Teirilä, L., Lappalainen, J., Rajamäki, K., Baumann, M. H., Öörni, K., Wolff, H., Kovanen, P. T., Matikainen, S., & Eklund, K. K. (2011). Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. The Journal of Immunology, 186, 6119–6128.
Pelegrin, P., Barroso-Gutierrez, C., & Surprenant, A. (2008). P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. The Journal of Immunology, 180, 7147–7157.
Lee, B. H., Hwang, D. M., Palaniyar, N., Grinstein, S., Philpott, D. J., & Hu, J. (2012). Activation of P2X7 receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS ONE, 7, e35812.
Clayton, A., Al-Taei, S., Webber, J., Mason, M. D., & Tabi, Z. (2011). Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. The Journal of Immunology, 187, 676–683.
Zhai, X., Chen, K., Yang, H., Li, B., Zhou, T., Wang, H., Zhou, H., Chen, S., Zhou, X., & Wei, X. (2021). Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury. Journal of Nanobiotechnology, 19, 1–20.
Oliveira-Giacomelli, Á., Albino, C. M., de Souza, H. D. N., Corrêa-Velloso, J., de Jesus Santos, A. P., Baranova, J., & Ulrich, H. (2019). P2Y6 and P2X7 receptor antagonism exerts neuroprotective/neuroregenerative effects in an animal model of Parkinson’s disease. Frontiers in Cellular Neuroscience, 13, 476.
Quintas, C., Vale, N., Gonçalves, J., & Queiroz, G. (2018). Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Frontiers in Pharmacology, 9, 418.
Pérez-Sen, R., Queipo, M. J., Morente, V., Ortega, F., Delicado, E. G., & Miras-Portugal, M. T. (2015). Neuroprotection mediated by P2Y13 nucleotide receptors in neurons. Computational and Structural Biotechnology Journal, 13, 160–168.
Maeda, J., Minamihisamatsu, T., Shimojo, M., Zhou, X., Ono, M., Matsuba, Y., Ji, B., Ishii, H., Ogawa, M., & Akatsu, H. (2021). Distinct microglial response against Alzheimer’s amyloid and tau pathologies characterized by P2Y12 receptor. Brain Communications, 3, fcab011.
Schilling, U., Dingemanse, J., & Ufer, M. (2020). Pharmacokinetics and pharmacodynamics of approved and investigational P2Y12 receptor antagonists. Clinical Pharmacokinetics, 59, 545–566.
Merighi, S., Travagli, A., Nigro, M., Pasquini, S., Cappello, M., Contri, C., Varani, K., Vincenzi, F., Borea, P. A., & Gessi, S. (2023). Caffeine for prevention of Alzheimer’s disease: Is the A2A adenosine receptor its target? Biomolecules, 13, 967.
Siniscalco, D., Giordano, C., Galderisi, U., Luongo, L., de Novellis, V., Rossi, F., & Maione, S. (2011). Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Frontiers in Integrative Neuroscience, 5, 79.
Ti, D., Hao, H., Tong, C., Liu, J., Dong, L., Zheng, J., Zhao, Y., Liu, H., Fu, X., & Han, W. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. Journal of Translational Medicine, 13, 1–14.
Kim, J., & Hematti, P. (2009). Mesenchymal stem cells convert human macrophages to a novel type of alternatively activated macrophages. Blood, 114, 3632.
Chen, B., Ni, Y., Liu, J., Zhang, Y., & Yan, F. (2018). Bone marrow-derived mesenchymal stem cells exert diverse effects on different macrophage subsets. Stem Cells International, 2018, 8348121.
Pu, J., Zhang, Y., Wang, A., Qin, Z., Zhuo, C., Li, W., Xu, Z., Tang, Q., Wang, J., & Wei, H. (2021). ADORA2A-AS1 restricts hepatocellular carcinoma progression via binding HuR and repressing FSCN1/AKT axis. Frontiers in Oncology, 11, 754835.
Acknowledgements
HU acknowledges grant support for his work on purinergic signaling by the São Paulo Research Foundation [(FAPESP) Project No. 2018/07366-4 and 2012/50880-4] and the National Institute of Science and Technology in Regenerative Medicine (INCT-REGENERA), Brazil, as well as by the National Council of Scientific and Technological Development (CNPq Project No. 406396/2021 and 308012/2021-6). YN was supported by FAPESP fellowship (Project No. 2017/23604-0 and 2015/14343-2) and NT acknowledges a doctoral fellowship from FAPESP (Project No 2020/10725-6). H.U. was awarded with a Fulbright Chair in Global Health Brazil at Rutgers New Jersey Medical School.
Author information
Authors and Affiliations
Contributions
YN conducted the experiments, analyzed the data, provided the concepts, and prepare the first draft of the manuscript. LSS cultured MSCs, conducted experiments, wrote the manuscript, and analyzed the data. NT wrote the manuscript and performed data analyses. YK wrote the manuscript and performed data analyses. MZR provided intellectual input into the overall design, edited the paper and analyzed the data. NS provided reagents, edited the manuscript, and provided intellectual input. PR wrote and edited the final version of the manuscript and is responsible for the ethics of using human bone marrow aspirates, and provided the concepts for the experimental design. HU wrote and edited the final version of the manuscript and provided the conceptualized overall hypothesis.
Corresponding authors
Ethics declarations
Ethics Committee Approval
Rutgers Institutional Review Board approved this study (see method section).
Consent to Participate
Participating subjects signed the informed consent forms (see method section).
Consent for Publication
All authors have read the final version of this manuscript and agree with its publication.
Conflicts of Interest/Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naaldijk, Y., Sherman, L.S., Turrini, N. et al. Mesenchymal Stem Cell—Macrophage Crosstalk Provides Specific Exosomal Cargo to Direct Immune Response Licensing of Macrophages during Inflammatory Responses. Stem Cell Rev and Rep 20, 218–236 (2024). https://doi.org/10.1007/s12015-023-10612-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10612-3