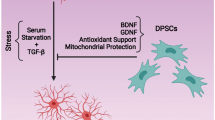
Abstract
Background
Transplantation of stem cells for treating neurodegenerative disorders is a promising future therapeutic approach. However, the molecular mechanism underlying the neuronal differentiation of dental pulp-derived stem cells (DPSC) remains inadequately explored. The current study aims to define the regulatory role of KLF2 (Kruppel-like factor 2) during the neural differentiation (ND) of DPSC.
Methods
We first investigated the transcriptional and translational expression of KLF2, autophagy, and mitophagy-associated markers during the ND of DPSC by using quantitative RT-PCR and western blot methods. After that, we applied the chemical-mediated loss- and gain-of-function approaches using KLF2 inhibitor, GGPP (geranylgeranyl pyrophosphate), and KLF2 activator, GGTI-298 (geranylgeranyl transferase inhibitor-298) to delineate the role of KLF2 during ND of DPSC. The western blot, qRT-PCR, and immunocytochemistry were performed to determine the molecular changes during ND after KLF2 deficiency and KLF2 sufficiency. We also analyzed the oxygen consumption rate (OCR) and the extracellular acidification rate (ECAR) using the Seahorse XFe24 analyzer.
Results
Our study demonstrated that the expression level of KLF2, autophagy, and mitophagy-associated markers were significantly elevated during the ND of DPSC. Next, we found that the KLF2 inhibitor, GGPP significantly reduced the ND of DPSC. Inversely, KLF2 overexpression accelerated the molecular phenomenon of DPSC’s commitment towards ND, indicating the crucial role of KLF2 in neurogenesis. Moreover, we found that the KLF2 positively regulated autophagy, mitophagy, and the Wnt5a signaling pathway during neurogenesis. Seahorse XFe24 analysis revealed that the ECAR and OCR parameters were significantly increased during ND, and inhibition of KLF2 marginally reversed them towards DPSC’s cellular bioenergetics. However, KLF2 overexpression shifted the cellular energy metabolism toward the quiescent stage.
Conclusion
Collectively, our findings provide the first evidence that the KLF2 critically regulates the neurogenesis of DPSC by inducing autophagy and mitophagy.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- DPSC:
-
Dental pulp-derived stem cells
- ECAR:
-
Extracellular acidification rate
- GGPP:
-
Geranylgeranyl pyrophosphate
- GGTI-298:
-
Geranylgeranyl transferase inhibitor-298
- HBSS:
-
Hanks’ balanced salt solution
- KLF2:
-
Krüppel-like factor 2
- KLFs:
-
Krüppel-like factors
- MEME:
-
Minimum essential medium eagle
- MSCs:
-
Mesenchymal stem cells
- ND:
-
Neural differentiation
- NDM:
-
Neural differentiation medium
- OCR:
-
Oxygen consumption rate
- ROS:
-
Reactive oxygen species
References
Gao, H. M., & Hong, J. S. (2008). Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends In Immunology, 29(8), 357–365.
Lindvall, O., Kokaia, Z., & Martinez-Serrano, A. (2004). Stem cell therapy for human neurodegenerative disorders–how to make it work. Nature medicine, 10(7), S42–S50.
Joyce, N., Annett, G., Wirthlin, L., Olson, S., Bauer, G., & Nolta, J. A. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative medicine, 5(6), 933–946.
Reboussin, É., Buffault, J., Brignole-Baudouin, F., Goazigo, R. L., Riancho, L., Olmiere, C., et al. (2022). Evaluation of neuroprotective and immunomodulatory properties of mesenchymal stem cells in an ex vivo retinal explant model. Journal of neuroinflammation, 19(1), 1–17.
Kim, Y. J., Park, H. J., Lee, G., Bang, O. Y., Ahn, Y. H., Joe, E., et al. (2009). Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia, 57(1), 13–23.
Giacomelli, E., Vahsen, B. F., Calder, E. L., Xu, Y., Scaber, J., Gray, E., et al. (2022). Human stem cell models of neurodegeneration: From basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell, 29(1), 11–35.
Puranik, N., Arukha, A. P., Yadav, S. K., Yadav, D., & Jin, J. O. (2022). Exploring the role of stem cell therapy in treating neurodegenerative diseases: Challenges and current perspectives. Current Stem Cell Research & Therapy, 17(2), 113–125.
Rolph, D. N., Deb, M., Kanji, S., Greene, C. J., Das, M., Joseph, M. (2018). Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical wnt signaling. Biochim Biophys Acta Mol Basis Dis. :165314.
Ledesma-Martínez, E., Mendoza-Núñez, V. M., & Santiago-Osorio, E. (2016). Mesenchymal stem cells derived from dental pulp: a review. Stem cells international. ;2016.
Lan, X., Sun, Z., Chu, C., Boltze, J., & Li, S. (2019). Dental pulp stem cells: An attractive alternative for cell therapy in ischemic stroke. Frontiers in neurology, 10, 824.
Mortada, I., Mortada, R., & Al Bazzal, M. (2018). Dental pulp stem cells and the management of neurological diseases: An update. Journal of Neuroscience Research, 96(2), 265–272.
Heng, B. C., Lim, L. W., Wu, W., & Zhang, C. (2016). An overview of protocols for the neural induction of dental and oral stem cells in vitro. Tissue Engineering Part B: Reviews, 22(3), 220–250.
Wang, F., Jia, Y., Liu, J., Zhai, J., Cao, N., Yue, W., et al. (2017). Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biology International, 41(6), 639–650.
Sharma, Y., Shobha, K., Sundeep, M., Pinnelli, V. B., Parveen, S., & Dhanushkodi, A. (2022). Neural basis of Dental Pulp Stem cells and its potential application in Parkinson’s disease. CNS & neurological Disorders-Drug targets (formerly current drug Targets-CNS & neurological Disorders). ;21(1):62–76.
Mead, B., Logan, A., Berry, M., Leadbeater, W., & Scheven, B. A. (2017). Concise review: Dental pulp stem cells: A novel cell therapy for retinal and central nervous system repair. Stem Cells, 35(1), 61–67.
Abeysinghe, H. C., Bokhari, L., Quigley, A., Choolani, M., Chan, J., Dusting, G. J., et al. (2015). Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke. Stem Cell Research & Therapy, 6, 186.
Bonaventura, G., Munafo, A., Bellanca, C. M., La Cognata, V., Iemmolo, R., Attaguile, G. A. (2021). Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells. ;10(8).
Osathanon, T., Sawangmake, C., Nowwarote, N., & Pavasant, P. (2014). Neurogenic differentiation of human dental pulp stem cells using different induction protocols. Oral diseases, 20(4), 352–358.
Chang, C. C., Chang, K. C., Tsai, S. J., Chang, H. H., & Lin, C. P. (2014). Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. Journal of the Formosan Medical Association, 113(12), 956–965.
Heng, B. C., Jiang, S., Yi, B., Gong, T., Lim, L. W., & Zhang, C. (2019). Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Archives of oral biology, 102, 26–38.
Chang, N. C. (2020). Autophagy and stem cells: Self-eating for self-renewal. Frontiers in Cell and Developmental Biology, 8, 138.
Onishi, M., Yamano, K., Sato, M., Matsuda, N., & Okamoto, K. (2021). Molecular mechanisms and physiological functions of mitophagy. The EMBO journal, 40(3), e104705.
Lin, Q., Chen, J., Gu, L., Dan, X., Zhang, C., & Yang, Y. (2021). New insights into mitophagy and stem cells. Stem cell research & therapy, 12(1), 1–14.
Fleming, A., & Rubinsztein, D. C. (2020). Autophagy in neuronal development and plasticity. Trends in neurosciences, 43(10), 767–779.
Doxaki, C., & Palikaras, K. (2021). Neuronal mitophagy: Friend or foe? Frontiers in cell and developmental biology, 8, 611938.
Sotthibundhu, A., Muangchan, P., Phonchai, R., Promjantuek, W., Chaicharoenaudomrung, N., Kunhorm, P., et al. (2021). Autophagy promoted neural differentiation of human placenta-derived mesenchymal stem cells. in vivo, 35(5), 2609–2620.
Das, H., Kumar, A., Lin, Z., Patino, W. D., Hwang, P. M., Feinberg, M. W., et al. (2006). Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A, 103(17), 6653–6658.
Rolph, D., & Das, H. (2020). Transcriptional regulation of Osteoclastogenesis: The emerging role of KLF2. Frontiers In Immunology, 11, 937.
Jha, P., & Das, H. (2017). KLF2 in regulation of NF-kappaB-mediated Immune cell function and inflammation. International Journal Of Molecular Sciences. ;18(11).
Sen-Banerjee, S., Mir, S., Lin, Z., Hamik, A., Atkins, G. B., Das, H., et al. (2005). Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation, 112(5), 720–726.
Laha, D., Deb, M., & Das, H. (2019). KLF2 (kruppel-like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy, 15(12), 2063–2075.
Winkelmann, R., Sandrock, L., Porstner, M., Roth, E., Mathews, M., Hobeika, E., et al. (2011). B cell homeostasis and plasma cell homing controlled by Krüppel-like factor 2. Proceedings of the National Academy of Sciences, 108(2), 710–715.
Feinberg, M. W., Lin, Z., Fisch, S., & Jain, M. K. (2004). An emerging role for Kruppel-like factors in vascular biology. Trends In Cardiovascular Medicine, 14(6), 241–246.
Maity, J., Barthels, D., Sarkar, J., Prateeksha, P., Deb, M., Rolph, D., et al. (2022). Ferutinin induces osteoblast differentiation of DPSCs via induction of KLF2 and autophagy/mitophagy. Cell Death And Disease, 13(5), 1–12.
Wu, F., & Li, C. (2022). KLF2 up-regulates IRF4/HDAC7 to protect neonatal rats from hypoxic-ischemic brain damage. Cell death discovery, 8(1), 1–10.
Zhang, J. H. (2013). Autophagy and mitophagy in cellular damage control. Redox Biology, 1(1), 19–23.
Parmar, K. M., Nambudiri, V., Dai, G., Larman, H. B., Gimbrone, M. A. Jr., & Garcia-Cardena, G. (2005). Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. Journal Of Biological Chemistry, 280(29), 26714–26719.
Maity, J., Deb, M., Greene, C., & Das, H. (2020). KLF2 regulates dental pulp-derived stem cell differentiation through the induction of mitophagy and altering mitochondrial metabolism. Redox Biology, 36, 101622.
Kondo, T., Matsuoka, A. J., Shimomura, A., Koehler, K. R., Chan, R. J., Miller, J. M., et al. (2011). Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem cells, 29(5), 836–846.
Lorzadeh, S., Kohan, L., Ghavami, S., & Azarpira, N. (2021). Autophagy and the wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1868(3), 118926.
Ozgen, S., Krigman, J., Zhang, R., & Sun, N. (2022). Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases. Neural Regeneration Research, 17(4), 741.
Brunetti, D., Dykstra, W., Le, S., Zink, A., & Prigione, A. (2021). Mitochondria in neurogenesis: Implications for mitochondrial diseases. Stem Cells, 39(10), 1289–1297.
Kuntz, E. M., Baquero, P., Michie, A. M., Dunn, K., Tardito, S., Holyoake, T. L., et al. (2017). Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nature medicine, 23(10), 1234–1240.
Zheng, X., Boyer, L., Jin, M., Mertens, J., Kim, Y., Ma, L., et al. (2016). Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. elife, 5, e13374.
Birket, M. J., Orr, A. L., Gerencser, A. A., Madden, D. T., Vitelli, C., Swistowski, A., et al. (2011). A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. Journal Of Cell Science, 124(Pt 3), 348–358.
Birket, M. J., Orr, A. L., Gerencser, A. A., Madden, D. T., Vitelli, C., Swistowski, A., et al. (2011). A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. Journal of cell science, 124(3), 348–358.
Zhang, C., Skamagki, M., Liu, Z., Ananthanarayanan, A., Zhao, R., Li, H., et al. (2017). Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell reports, 21(8), 2058–2065.
Acknowledgements
We are thankful to Zijuan Liu, from TTUHSC core facility for her help in procuring some of the confocal images. The core facility is being supported by a Core Facility Support Award (grant number RP200572) from the Cancer Prevention and Research Institute of Texas (CPRIT) to the Imaging Core, Texas Tech University Health Sciences Center at Amarillo.
Funding
This work was supported in part by National Institutes of Health grants, R01AR068279 (NIAMS), STTR R42EY031196 (NEI), and STTR 1R41AG057242 (NIA). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception, design, and manuscript writing: PP and HD. Acquisition of data: PP, PN, MD, DB Analysis and interpretation of data: PP and HD.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Authors provide the consent for publication.
Competing Interests
Authors do not have any potential conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prateeksha, P., Naidu, P., Das, M. et al. KLF2 Regulates Neural Differentiation of Dental Pulp-derived Stem Cells by Modulating Autophagy and Mitophagy. Stem Cell Rev and Rep 19, 2886–2900 (2023). https://doi.org/10.1007/s12015-023-10607-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10607-0