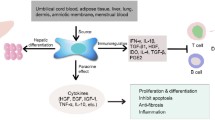
Abstract
Liver fibrosis was initially considered to be an irreversible process which will eventually lead to the occurrence of liver cancer. So far there has been no effective therapeutic approach to treat liver fibrosis although scientists have put tremendous efforts into the underlying mechanisms of this disease. Therefore, in-depth research on novel and safe treatments of liver fibrosis is of great significance to human health. Pluripotent stem cells (PSCs) play important roles in the study of liver fibrosis due to their unique features in self-renewal ability, pluripotency, and paracrine function. This article mainly reviews the applications of PSCs in the study of liver fibrosis in recent years. We discuss the role of PSC-derived liver organoids in the study of liver fibrosis, and the latest research advances on the differentiation of PSCs into hepatocytes or macrophages. We also highlight the importance of exosomes of PSCs for the treatment of liver fibrosis.
Graphical Abstract



Similar content being viewed by others
Data Availability
Not applicable.
References
Dewidar, B., et al. (2019). TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells, 8(11), 1419.
Bataller, R., & Brenner, D. A. (2005). Liver fibrosis. The Journal of Clinical Investigation, 115(2), 209–218.
Friedman, S. L. (2008). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews, 88(1), 125–172.
Trivedi, P., Wang, S., & Friedman, S. L. (2021). The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metabolism, 33(2), 242–257.
Arroyo, N., et al. (2021). GATA4 induces liver fibrosis regression by deactivating hepatic stellate cells. JCI Insight, 6(23), e150059.
Troeger, J. S., et al. (2012). Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology, 143(4), 1073–83 e22.
Gao, J., et al. (2020). Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. Journal of Hepatology, 73(5), 1144–1154.
Friedman, S. L., & Pinzani, M. (2022). Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology, 75(2), 473–488.
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell, 27(4), 523–531.
Thomson, J. A., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Moriya, K., et al. (2007). Embryonic stem cells develop into hepatocytes after intrasplenic transplantation in CCl4-treated mice. World Journal of Gastroenterology, 13(6), 866–873.
Moriya, K., et al. (2008). Embryonic stem cells reduce liver fibrosis in CCl4-treated mice. International Journal of Experimental Pathology, 89(6), 401–409.
Lai, X., et al. (2022). Generation of functionally competent hepatic stellate cells from human stem cells to model liver fibrosis in vitro. Stem Cell Reports, 17(11), 2531–2547.
Takahashi, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Yu, J., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Park, I. H., et al. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451(7175), 141–146.
Espejel, S., et al. (2010). Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. The Journal of Clinical Investigation, 120(9), 3120–3126.
Coll, M., et al. (2018). Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell, 23(1), 101-113 e7.
Kiso, A., et al. (2020). Tolloid-Like 1 negatively regulates hepatic differentiation of human induced pluripotent stem cells through transforming growth factor beta signaling. Hepatology Communications, 4(2), 255–267.
Koui, Y., et al. (2021). Development of human iPSC-derived quiescent hepatic stellate cell-like cells for drug discovery and in vitro disease modeling. Stem Cell Reports, 16(12), 3050–3063.
Vallverdu, J., et al. (2021). Directed differentiation of human induced pluripotent stem cells to hepatic stellate cells. Nature Protocols, 16(5), 2542–2563.
Corro, C., Novellasdemunt, L., & Li, V. S. W. (2020). A brief history of organoids. American Journal of Physiology Cell Physiology, 319(1), C151–C165.
Lancaster, M. A., & Huch, M. (2019). Disease modelling in human organoids. Disease Models & Mechanisms, 12(7), dmm039347.
Lancaster, M. A., & Knoblich, J. A. (2014). Organogenesis in a dish: Modeling development and disease using organoid technologies. Science, 345(6194), 1247125.
Nuciforo, S., & Heim, M. H. (2021). Organoids to model liver disease. JHEP Rep, 3(1), 100198.
Brovold, M., Keller, D., & Soker, S. (2020). Differential fibrotic phenotypes of hepatic stellate cells within 3D liver organoids. Biotechnology and Bioengineering, 117(8), 2516–2526.
Wang, S., et al. (2019). Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Research, 29(12), 1009–1026.
Tsuchida, T., et al. (2018). A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. Journal of Hepatology, 69(2), 385–395.
Bruck, R., et al. (2001). Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology, 33(2), 379–386.
Tolba, R., et al. (2015). Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Laboratory Animals, 49(1 Suppl), 59–69.
van Os, E. A., et al. (2022). Modelling fatty liver disease with mouse liver-derived multicellular spheroids. Biomaterials, 290, 121817.
Wang, Y., et al. (2019). Splenectomy promotes macrophage polarization in a mouse model of Concanavalin A- (ConA-) induced liver fibrosis. BioMed Research International, 2019, 5756189.
Tsukamoto, H., Gaal, K., & French, S. W. (1990). Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: Status report. Hepatology, 12(3 Pt 1), 599–608.
Li, R., et al. (2018). Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. International Journal of Biological Sciences, 14(11), 1411–1425.
Liu, J. Y., et al. (2021). Multiparameter magnetic resonance imaging of liver fibrosis in a bile duct ligation mouse model. World Journal of Gastroenterology, 27(47), 8156–8165.
Bao, Y. L., et al. (2021). Animal and organoid models of liver fibrosis. Frontiers in Physiology, 12, 666138.
Wu, X., et al. (2023). Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids. Cell Regeneration, 12(1), 6.
Ouchi, R., et al. (2019). Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metabolism, 30(2), 374-384 e6.
Shinozawa, T., et al. (2021). High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology, 160(3), 831-846 e10.
Han, L., et al. (2020). Single cell transcriptomics identifies a signaling network coordinating endoderm and mesoderm diversification during foregut organogenesis. Nature Communications, 11(1), 4158.
McCracken, K. W., et al. (2017). Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature, 541(7636), 182–187.
Spence, J. R., et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 470(7332), 105–109.
Guan, Y., et al. (2017). Human hepatic organoids for the analysis of human genetic diseases. JCI Insight, 2(17), e94954.
Jiang, S., et al. (2022). Development of a high-throughput micropatterned agarose scaffold for consistent and reproducible hPSC-derived liver organoids. Biofabrication, 15(1), 015006.
Kim, H. J., et al. (2023). Generation of multilineage liver organoids with luminal vasculature and bile ducts from human pluripotent stem cells via modulation of Notch signaling. Stem Cell Research & Therapy, 14(1), 19.
Tang, X. Y., et al. (2022). Human organoids in basic research and clinical applications. Signal Transduction and Targeted Therapy, 7(1), 168.
Sgodda, M., et al. (2017). A scalable approach for the generation of human pluripotent stem cell-derived hepatic organoids with sensitive hepatotoxicity features. Stem Cells Dev, 26(20), 1490–1504.
Cheng, W., et al. (2022). Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Science of the Total Environment, 806(Pt 1), 150328.
Cheng, W., et al. (2023). Combined effect of polystyrene microplastics and bisphenol A on the human embryonic stem cells-derived liver organoids: The hepatotoxicity and lipid accumulation. Science of The Total Environment, 854, 158585.
Molenaar, M. R., Vaandrager, A. B., & Helms, J. B. (2017). Some lipid droplets are more equal than others: different metabolic lipid droplet pools in hepatic stellate cells. Lipid Insights, 10, 1178635317747281.
Jing, X. Y., et al. (2013). Roles of the lipid metabolism in hepatic stellate cells activation big up tri, open. Chinese Medical Sciences Journal, 28(4), 233–236.
Hendriks, D., Clevers, H., & Artegiani, B. (2020). CRISPR-Cas tools and their application in genetic engineering of human stem cells and organoids. Cell Stem Cell, 27(5), 705–731.
Manghwar, H., et al. (2019). CRISPR/Cas system: Recent advances and future prospects for genome editing. Trends in Plant Science, 24(12), 1102–1125.
Gupta, D., et al. (2019). CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sciences, 232, 116636.
Oh, H. T., et al. (2022). CD133-Src-TAZ signaling stimulates ductal fibrosis following DDC diet-induced liver injury. Journal of Cellular Physiology, 237(12), 4504–4516.
Artegiani, B., et al. (2020). Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nature Cell Biology, 22(3), 321–331.
Tsunoda, T., et al. (2019). Loss of fibrocystin promotes interleukin-8-dependent proliferation and CTGF production of biliary epithelium. Journal of Hepatology, 71(1), 143–152.
Alfaifi, M., et al. (2018). Mesenchymal stromal cell therapy for liver diseases. Journal of Hepatology, 68(6), 1272–1285.
Yang, X., et al. (2023). Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment. Cellular & Molecular Immunology, 20(6), 583–599.
Kim, J., et al. (2021). sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Molecular Therapy, 29(4), 1471–1486.
Sauer, V., et al. (2014). Induced pluripotent stem cells as a source of hepatocytes. Current Pathobiology Reports, 2(1), 11–20.
Chen, Y., et al. (2021). Treatment of alpha-1 antitrypsin deficiency using hepatic-specified cells derived from human-induced pluripotent stem cells. American Journal of Translational Research, 13(4), 2710–2716.
Huang, P., et al. (2011). Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature, 475(7356), 386–389.
Park, S., et al. (2019). The therapeutic potential of induced hepatocyte-like cells generated by direct reprogramming on hepatic fibrosis. Stem Cell Research & Therapy, 10(1), 21.
Lee, C. A., et al. (2018). Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine. Journal of Molecular Medicine (Berlin, Germany), 96(6), 469–481.
Choi, J. S., et al. (2020). HGF and IL-10 expressing ALB::GFP reporter cells generated from iPSCs show robust anti-fibrotic property in acute fibrotic liver model. Stem Cell Research & Therapy, 11(1), 332.
Aghadi, M., Elgendy, R., & Abdelalim, E. M. (2022). Loss of FOXA2 induces ER stress and hepatic steatosis and alters developmental gene expression in human iPSC-derived hepatocytes. Cell Death & Disease, 13(8), 713.
Moore, J. K., et al. (2015). Phenotypic and functional characterization of macrophages with therapeutic potential generated from human cirrhotic monocytes in a cohort study. Cytotherapy, 17(11), 1604–1616.
Haideri, S. S., et al. (2017). Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury. NPJ Regen Med, 2, 14.
Pouyanfard, S., et al. (2021). Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis. Stem Cells, 39(12), 1701–1717.
Cheng, D., et al. (2021). Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver International, 41(10), 2279–2294.
Kupffer, C. (1876). Ueber Sternzellen der Leber. Archiv für mikroskopische Anatomie, 12(1), 353–358.
Tacke, F., & Zimmermann, H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. Journal of Hepatology, 60(5), 1090–1096.
Tasnim, F., et al. (2019). Generation of mature kupffer cells from human induced pluripotent stem cells. Biomaterials, 192, 377–391.
Lian, Q., et al. (2010). Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation, 121(9), 1113–1123.
Lian, Q., et al. (2022). Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nature Communications, 13(1), 2028.
Kou, M., et al. (2022). Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death & Disease, 13(7), 580.
Thakur, A., et al. (2022). The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein & Cell, 13(9), 631–654.
Jeske, R., et al. (2020). Human pluripotent stem cell-derived extracellular vesicles: Characteristics and applications. Tissue Engineering. Part B, Reviews, 26(2), 129–144.
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19(4), 213–228.
Valadi, H., et al. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659.
Meckes, D. G., Jr., et al. (2010). Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America, 107(47), 20370–20375.
Cheng, L., & Hill, A. F. (2022). Therapeutically harnessing extracellular vesicles. Nature Reviews. Drug Discovery, 21(5), 379–399.
Pegtel, D. M., & Gould, S. J. (2019). Exosomes. Annual Review of Biochemistry, 88, 487–514.
Colombo, M., Raposo, G., & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289.
Ying, W., et al. (2021). MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metabolism, 33(4), 781-790 e5.
Li, C., et al. (2021). Cancer associated-fibroblast-derived exosomes in cancer progression. Molecular Cancer, 20(1), 154.
Guo, M., et al. (2020). Microglial exosomes facilitate alpha-synuclein transmission in Parkinson’s disease. Brain, 143(5), 1476–1497.
Zhang, T., et al. (2021). The emerging role of exosomes in Alzheimer’s disease. Ageing Research Reviews, 68, 101321.
Szabo, G. (2021). Exosomes and MicroRNA-223 at the Intersection of Inflammation and Fibrosis in NAFLD. Hepatology, 74(1), 5–8.
Bi, Y., et al. (2022). Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells. Stem Cell Research & Therapy, 13(1), 449.
Taheri, B., et al. (2019). Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. Journal of Cellular Physiology, 234(6), 8455–8464.
Liu, S., et al. (2019). Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells, 37(6), 779–790.
Povero, D., et al. (2019). Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight, 5(14),e125652.
Wang, N., et al. (2021). 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFbetaRII-SMADS pathway. J Nanobiotechnology, 19(1), 437.
Villa-Diaz, L. G., et al. (2012). Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells, 30(6), 1174–1181.
McGrath, M., et al. (2019). GMP-compatible and xeno-free cultivation of mesenchymal progenitors derived from human-induced pluripotent stem cells. Stem Cell Research & Therapy, 10(1), 11.
Zhang, J., et al. (2021). Induced pluripotent stem cell-derived mesenchymal stem cells hold lower heterogeneity and great promise in biological research and clinical applications. Frontiers in Cell and Developmental Biology, 9, 716907.
Barberi, T., et al. (2005). Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Medicine, 2(6), e161.
Olivier, E. N., Rybicki, A. C., & Bouhassira, E. E. (2006). Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells, 24(8), 1914–1922.
Sabapathy, V., & Kumar, S. (2016). hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. Journal of Cellular and Molecular Medicine, 20(8), 1571–1588.
Lotfinia, M., et al. (2016). Effect of secreted molecules of human embryonic stem cell-derived mesenchymal stem cells on acute hepatic failure model. Stem Cells and Development, 25(24), 1898–1908.
Spitzhorn, L. S., et al. (2019). Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Research & Therapy, 10(1), 100.
Qiu, C., et al. (2005). Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Experimental Hematology, 33(12), 1450–1458.
Hong, K. S., et al. (2015). A porous membrane-mediated isolation of mesenchymal stem cells from human embryonic stem cells. Tissue Engineering. Part C, Methods, 21(3), 322–329.
Liu, Y., et al. (2012). One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS ONE, 7(3), e33225.
Chen, Y. S., et al. (2012). Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Translational Medicine, 1(2), 83–95.
Jiang, B., et al. (2019). Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics, 9(22), 6587–6600.
Himeno, T., et al. (2013). Mesenchymal stem cell-like cells derived from mouse induced pluripotent stem cells ameliorate diabetic polyneuropathy in mice. BioMed Research International, 2013, 259187.
Nachlas, A. L. Y., et al. (2018). Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells. Acta Biomaterialia, 71, 235–246.
Gong, M., et al. (2011). Immortalized mesenchymal stem cells: An alternative to primary mesenchymal stem cells in neuronal differentiation and neuroregeneration associated studies. Journal of Biomedical Science, 18(1), 87.
Wu, Q., et al. (2020). The sialylation profile of IgG determines the efficiency of antibody directed osteogenic differentiation of iMSCs by modulating local immune responses and osteoclastogenesis. Acta Biomaterialia, 114, 221–232.
Harding, J., & Mirochnitchenko, O. (2014). Preclinical studies for induced pluripotent stem cell-based therapeutics. Journal of Biological Chemistry, 289(8), 4585–4593.
Kim, S., et al. (2018). Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. International Journal of Molecular Sciences, 19(10), 3119.
Zhu, Y., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research & Therapy, 8(1), 64.
Du, Y., et al. (2017). Exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and Sphingosine-1-Phosphate signaling pathway. Cellular Physiology and Biochemistry, 43(2), 611–625.
Mardpour, S., et al. (2018). Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. Journal of Cellular Physiology, 233(12), 9330–9344.
Otsuka, R., et al. (2020). Immune reaction and regulation in transplantation based on pluripotent stem cell technology. Inflammation and Regeneration, 40, 12.
Haque, R., et al. (2012). Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. The Journal of Immunology, 189(3), 1228–1236.
Wang, D., et al. (2019). Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death & Disease, 10(6), 443.
Rong, X., et al. (2020). Antler stem cells as a novel stem cell source for reducing liver fibrosis. Cell and Tissue Research, 379(1), 195–206.
Le Berre, C., et al. (2020). Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology, 158(1), 76-94 e2.
Bannigan, P., et al. (2021). Machine learning directed drug formulation development. Advanced Drug Delivery Reviews, 175, 113806.
Funding
Wu Q was supported by Macau Science and Technology Development Fund (file number 0072/2019/A2). Tam PKH was supported by Research Grant Council Theme-based Research Scheme 2021–22 (T12-712/21-R), Health and Medical Research Fund (HMRF) (Project No. 08192376).
Author information
Authors and Affiliations
Contributions
Ma L and Wu Q completed most of the writing; Wu Q and Tam PKH revised the manuscript; Wu Q and Tam PKH conceived the idea, revised, and proofread the paper.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Wu, Q. & Tam, P.KH. The Current Proceedings of PSC-Based Liver Fibrosis Therapy. Stem Cell Rev and Rep 19, 2155–2165 (2023). https://doi.org/10.1007/s12015-023-10592-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10592-4