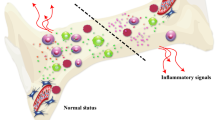
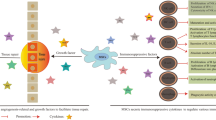
Abstract
Lupus is known as a systemic immune-mediated disorder. Like other diseases in this category, its cause and definitive treatment remain unknown. Gold standard therapies, which mainly include immunosuppressive agents, have been able to have therapeutic effects on patients. However, a significant percentage of cases still do not respond to this kind of treatment, resulting in death from complications. Recently, a new source of non-hematopoietic cells, mesenchymal stem/stromal cells (MSCs), with the potency to re-establishment immune homeostasis and tissue regeneration, has been wildly used in both primary and clinical research. One of the remarkable features of MSCs is their anti-inflammatory and immunosuppressive properties and stimulating tissue differentiation programs. Under the influence of background signals, MSCs migrate to inflammatory bioactive substances and then regulate overactive immune responses to restore immune tolerance. MSCs have shown a two-way interaction with most immunocytes, which plays a significant role in resolving sterile inflammation. Restricting the entry of inflamed cells into the site of inflammation and re-educated infiltrated cells to achieve a tolerant phenotype have been reported as mechanisms of MSCs in tissue repair. Stimulation of the endogenous and tissue-dwelling stem cells in addition to releasing immunomodulatory agents, suggests MSCs transplantation as a potential modality in the treatment of future immune-mediated disorders.
Graphical abstract




Similar content being viewed by others
Data Availability
There is no data to share because the article is a review.
Abbreviations
- AD:
-
Adipose-derived
- BAX:
-
Bcl-2-associated X protein
- Bcl-2:
-
Bcell lymphoma 2
- BM:
-
Bone marrow
- C3aR:
-
C3a receptor
- C5aR:
-
C5a receptor
- CCL19:
-
chemokine (C-C motif) ligand 19
- CCR7:
-
C-Cchemokine receptor 7
- DC:
-
Dendritic cell
- dsDNA:
-
Double strand DNA
- FH:
-
Factor H
- HLA-G5:
-
Human leukocyte antigen-G5
- HSC:
-
Hematopoietic stem cells
- ICAM-1:
-
Intercellular adhesion molecule1
- IDO:
-
Indoleamine 2, 3-dioxygenase
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- IM:
-
Intramuscular
- IP:
-
Intraperitoneal
- IV:
-
Intravascular
- LN:
-
Lupus nephritis
- MBL:
-
Mannose-binding lectin
- MHC:
-
Major histocompatibility complex
- MSC:
-
Mesenchymal stem cell
- MQ:
-
Macrophage
- NEU:
-
Neutrophil
- NK:
-
Natural killer
- PGE2:
-
Prostaglandin E2
- PMN:
-
Polymorphonuclear
- SLE:
-
Systemic lupus erythematosus
- T cell:
-
T helper cell
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- Treg:
-
T regulatory cell
- TSG-6:
-
Tumour necrosis factor-stimulated gene 6 protein
- UC:
-
Umbilical cord
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Klein, A., Polliack, A., & Gafter-Gvili, A. (2018). Systemic lupus erythematosus and lymphoma: Incidence, pathogenesis and biology. Leukemia Research, 75, 45–49.
Yeoh, S.-A., Dias, S. S., & Isenberg, D. A. (2018). Advances in systemic lupus erythematosus. Medicine, 46(2), 84–92.
Turksen, K. (2020). Cell biology and translational medicine, Vol. 9. In Stem Cell-Based Therapeutic Approaches in Disease, Vol. 1288. Springer Nature.
Potdar, P., & Deshpande, S. (2013). Mesenchymal stem cell transplantation: New, avenues for stem cell therapies. The Journal of Transplantation Technologies & Research, 3(122), 2161–0991 1000122.
Yan, S., et al. (2014). MicroRNA regulation in systemic lupus erythematosus pathogenesis. Immune Network, 14(3), 138–148.
Zan, H., Tat, C., & Casali, P. (2014). MicroRNAs in lupus. Autoimmunity, 47(4), 272–285.
Fava, A., & Petri, M. (2019). Systemic lupus erythematosus: Diagnosis and clinical management. Journal of Autoimmunity, 96, 1–13.
Gatto, M., et al. (2019). New therapeutic strategies in systemic lupus erythematosus management. Nature Reviews Rheumatology, 15(1), 30–48.
Kuhn, A., et al. (2015). The diagnosis and treatment of systemic lupus erythematosus. Deutsches Ärzteblatt International, 112(25), 423.
Cras, A., et al. (2015). Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Research & Therapy, 17(1), 1–9.
Wang, D., & Sun, L. (2019). Chapter 7 - systemic lupus erythematosus. In X.-D. Chen (Ed.), A roadmap to non-hematopoietic stem cell-based therapeutics (pp. 143–172). Academic Press.
Marmont, A., et al. (1997). Autologous marrow stem cell transplantation for severe systemic lupus erythematosus of long duration. Lupus, 6(6), 545–548.
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650.
Le Blanc, K., et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. The Lancet, 371(9624), 1579–1586.
Nauta, A. J., & Fibbe, W. E. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood, The Journal of the American Society of Hematology, 110(10), 3499–3506.
Arribas, M. I., et al. (2012). Adipose cell-derived stem cells: Neurogenic and immunomodulatory potentials. Advances in Neuroimmune Biology, 3(1), 19–30.
Luz-Crawford, P., et al. (2019). Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Research & Therapy, 10(1), 1–13.
Wang, D., et al. (2011). Effect of allogeneic bone marrow–derived mesenchymal stem cells transplantation in a polyI: C-induced primary biliary cirrhosis mouse model. Clinical and Experimental Medicine, 11(1), 25–32.
Wang, M., et al. (2016). Intraperitoneal injection (IP), intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Scientific Reports, 6, 30696.
Krampera, M., et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells, 24(2), 386–398.
Liotta, F., et al. (2008). Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing notch signaling. Stem Cells, 26(1), 279–289.
Somoza, R., Correa, D., & Caplan, A. (2016). Roles for mesenchymal stem cells as medicinal signaling cells. Nature Protocols, 11.
Kunisaki, Y., et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 502(7473), 637–643.
D, S.d.B, Casamitjana, J., & Crisan, M. (2017). Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacology & Therapeutics, 171, 104–113.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name! Stem Cells Translational Medicine, 6(6), 1445–1451.
Sala, E., et al. (2015). Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology, 149(1), 163–176.e20.
Lee, R. H., et al. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 5(1), 54–63.
Caplan, A. I., & Correa, D. (2011). The MSC: An injury drugstore. Cell Stem Cell, 9(1), 11–15.
Kabat, M., et al. (2020). Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Translational Medicine, 9(1), 17–27.
Moll, G., et al. (2019). Intravascular mesenchymal stromal/stem cell therapy product diversification: Time for new clinical guidelines. Trends in Molecular Medicine, 25(2), 149–163.
Braid, L. R., et al. (2018). Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy, 20(2), 232–244.
Han, J. W., et al. (2016). Bone marrow-derived mesenchymal stem cells improve diabetic neuropathy by direct modulation of both angiogenesis and myelination in peripheral nerves. Cell Transplantation, 25(2), 313–326.
Francki, A., et al. (2016). Angiogenic properties of human placenta-derived adherent cells and efficacy in hindlimb ischemia. Journal of Vascular Surgery, 64(3), 746–756 e1.
Lee, H.-B., et al. (2019). Intramuscular Injection of Adipose Tissue Derived Stromal Vascular Fraction in Subjects with Poliomyelitis: Case Reports. International Journal of Stem Cell Research & Therapeutics, 1(1), 20–27.
Soria-Juan, B., et al. (2021). Efficacy and safety of intramuscular administration of allogeneic adipose tissue derived and expanded mesenchymal stromal cells in patients with critical limb ischemia and type 2 diabetes with no possibility of revascularization: Study protocol for a randomized controlled double-blind phase II clinical trial (The NOMA Trial). Trials, 22(1), 595.
Wu, S. C., et al. (2017). Safety and efficacy of intramuscular human placenta-derived mesenchymal stromal-like cells (cenplacel [PDA-002]) in patients who have a diabetic foot ulcer with peripheral arterial disease. International Wound Journal, 14(5), 823–829.
van Rhijn-Brouwer, F. C., et al. (2018). A randomised placebo-controlled double-blind trial to assess the safety of intramuscular administration of allogeneic mesenchymal stromal cells for digital ulcers in systemic sclerosis: The MANUS trial protocol. BMJ Open, 8(8), e020479.
Youd, M., et al. (2010). Allogeneic mesenchymal stem cells do not protect NZB× NZW F1 mice from developing lupus disease. Clinical and Experimental Immunology, 161(1), 176–186.
Sun, L., et al. (2014). Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. International Journal of Molecular Medicine, 34(4), 987–996.
Oh, J. Y., et al. (2014). Intraperitoneal infusion of mesenchymal stem/stromal cells prevents experimental autoimmune uveitis in mice. Mediators of Inflammation, 2014.
Nam, Y., et al. (2018). Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS One, 13(6), e0198740.
Wang, M., et al. (2016). Intraperitoneal injection (IP), intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Scientific Reports, 6(1), 1–13.
da Costa Gonçalves, F., et al. (2014). Intravenous vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World Journal of Gastroenterology: WJG, 20(48), 18228.
Petrou, P., et al. (2016). Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurology, 73(3), 337–344.
Bartholomew, A., et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology, 30(1), 42–48.
Figueroa, F. E., et al. (2012). Mesenchymal stem cell treatment for autoimmune diseases: A critical review. Biological Research, 45(3), 269–277.
Marigo, I., & Dazzi, F. The immunomodulatory properties of mesenchymal stem cells. In seminars in immunopathology. 2011. Springer.
Shi, Y., et al. (2012). How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology, 33(3), 136–143.
Carrion, F. A., & Figueroa, F. E. (2011). Mesenchymal stem cells for the treatment of systemic lupus erythematosus: Is the cure for connective tissue diseases within connective tissue? Stem Cell Research & Therapy, 2(3), 23.
Bosi, C., Lanzoni, G., & Pugliese, A. (2016). Clinical trials of mesenchymal stem cell transplantation in patients with type 1 diabetes and systemic lupus erythematosus: Is it time for larger studies. CellR4, 4(5), e2134.
Wen, L., et al. (2019). Prognostic factors for clinical response in systemic lupus erythematosus patients treated by allogeneic mesenchymal stem cells. Stem Cells International, 2019, 7061408.
Zhu, Y., & Feng, X. (2018). Genetic contribution to mesenchymal stem cell dysfunction in systemic lupus erythematosus. Stem Cell Research & Therapy, 9(1), 1–6.
Brandau, S., et al. (2010). Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. Journal of Leukocyte Biology, 88(5), 1005–1015.
Taghavi-Farahabadi, M., et al. (2020). Immunological Investigations, 1–16.
Maqbool, M., et al. (2011). Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biology International, 35(12), 1247–1251.
Mittal, S. K., et al. (2018). Mesenchymal stromal cells inhibit neutrophil effector functions in a murine model of ocular inflammation. Investigative Ophthalmology & Visual Science, 59(3), 1191–1198.
Hall, S. R., et al. (2013). Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells, 31(2), 397–407.
Le Blanc, K., & Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews. Immunology, 12(5), 383–396.
Munir, H., et al. (2016). Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS One, 11(5), e0155161.
English, K. (2013). Mechanisms of mesenchymal stromal cell immunomodulation. Immunology and Cell Biology, 91(1), 19–26.
Cai, B., et al. (2021). N2-polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: A missing piece of the bone regeneration puzzle. Advanced Science, 8(19), 2100584.
Hu, X., et al. (2014). Programming of the development of tumor-promoting neutrophils by mesenchymal stromal cells. Cellular Physiology and Biochemistry, 33(6), 1802–1814.
Raffaghello, L., et al. (2008). Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells, 26(1), 151–162.
Zhao, J., et al. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research, 115(7), 1205–1216.
Nakajima, H., et al. (2012). Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. Journal of Neurotrauma, 29(8), 1614–1625.
Anderson, P., et al. (2013). Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut, 62(8), 1131–1141.
Zhang, Z., et al. (2018). Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrology, Dialysis, Transplantation, 33(11), 2069–2069.
Braza, F., et al. (2016). Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells, 34(7), 1836–1845.
Liu, Y., et al. (2018). AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine, 36, 140–150.
Kim, J., & Hematti, P. (2009). Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Experimental Hematology, 37(12), 1445–1453.
Eggenhofer, E., & Hoogduijn, M. J. (2012). Mesenchymal stem cell-educated macrophages. Transplantation Research, 1(1), 12.
Takizawa, N., et al. (2017). Bone marrow-derived mesenchymal stem cells propagate immunosuppressive/anti-inflammatory macrophages in cell-to-cell contact-independent and-dependent manners under hypoxic culture. Experimental Cell Research, 358(2), 411–420.
Tang, X.-D., et al. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells, 35(7), 1849–1859.
Hidalgo-Garcia, L., et al. (2018). Can a conversation between mesenchymal stromal cells and macrophages solve the crisis in the inflamed intestine? Frontiers in Pharmacology, 9, 179.
Morrison, T. J., et al. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. American Journal of Respiratory and Critical Care Medicine, 196(10), 1275–1286.
Lopez-Santalla, M., et al. (2020). Cell therapy with mesenchymal stem cells induces an innate immune memory response that attenuates experimental colitis in the long term. Journal of Crohn's and Colitis, 14(10), 1424–1435.
Spaggiari, G. M., et al. (2006). Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood, 107(4), 1484–1490.
Sotiropoulou, P. A., et al. (2006). Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells, 24(1), 74–85.
Fan, Y., et al. (2019). Human fetal liver mesenchymal stem cell-derived Exosomes impair natural killer cell function. Stem Cells and Development, 28(1), 44–55.
Spaggiari, G. M., & Moretta, L. (2012). Mesenchymal stem cell-natural killer cell interactions. In Stem cells and Cancer stem cells (Vol. Volume 4, pp. 217–224). Springer.
Galland, S., et al. (2017). Tumor-derived mesenchymal stem cells use distinct mechanisms to block the activity of natural killer cell subsets. Cell Reports, 20(12), 2891–2905.
Spaggiari, G. M., & Moretta, L. (2013). Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunology and Cell Biology, 91(1), 27–31.
Bruno, S., Deregibus, M. C., & Camussi, G. (2015). The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunology Letters, 168(2), 154–158.
Petri, R. M., et al. (2017). Activated tissue-resident mesenchymal stromal cells regulate natural killer cell immune and tissue-regenerative function. Stem Cell Reports, 9(3), 985–998.
Deng, Y., et al. (2014). Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1. Stem Cells and Development, 23(17), 2080–2092.
Radmanesh, F., et al. (2020). The immunomodulatory effects of mesenchymal stromal cell-based therapy in human and animal models of systemic lupus erythematosus. IUBMB Life, 72(11), 2366–2381.
Chen, P., Huang, Y., & Womer, K. L. (2015). Effects of mesenchymal stromal cells on human myeloid dendritic cell differentiation and maturation in a humanized mouse model. Journal of Immunological Methods, 427, 100–104.
Spaggiari, G. M., et al. (2009). MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood, The Journal of the American Society of Hematology, 113(26), 6576–6583.
Djokic, J., Tomic, S., & Čolić, M. (2015). Cross-talk between mesenchymal stem/stromal cells and dendritic cells. Current Stem Cell Research & Therapy.
Chiesa, S., et al. (2011). Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proceedings of the National Academy of Sciences, 108(42), 17384–17389.
Liu, X., et al. (2012). Mesenchymal stem/stromal cells induce the generation of novel IL-10–dependent regulatory dendritic cells by SOCS3 activation. The Journal of Immunology, 189(3), 1182–1192.
Zhao, Z. G., et al. (2012). The characteristics and immunoregulatory functions of regulatory dendritic cells induced by mesenchymal stem cells derived from bone marrow of patient with chronic myeloid leukaemia. European Journal of Cancer, 48(12), 1884–1895.
Zhang, B., et al. (2009). Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2–dependent regulatory dendritic cell population. Blood, The Journal of the American Society of Hematology, 113(1), 46–57.
Lu, Z., et al. (2019). Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Research & Therapy, 10(1), 1–16.
Beyth, S., et al. (2005). Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood, 105(5), 2214–2219.
Dokić, J., et al. (2013). Mesenchymal stem cells from periapical lesions modulate differentiation and functional properties of monocyte-derived dendritic cells. European Journal of Immunology, 43(7), 1862–1872.
Obermajer, N., et al. (2011). Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood, 118(20), 5498–5505.
Liu, Y., et al. (2014). MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochemical and Biophysical Research Communications, 450(4), 1409–1415.
Li, Y. P., et al. (2008). Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the notch pathway. Journal of Immunology, 180(3), 1598–1608.
de Witte, S. F. H., et al. (2018). Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by Monocytic cells. Stem Cells, 36(4), 602–615.
Wynn, T. A., Chawla, A., & Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature, 496(7446), 445–455.
Wynn, T. A., & Vannella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 44(3), 450–462.
Deng, Y., et al. (2016). Umbilical cord-derived mesenchymal stem cells instruct monocytes towards an IL10-producing phenotype by secreting IL6 and HGF. Scientific Reports, 6(1), 1–9.
Selleri, S., et al. (2016). Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget, 7(21), 30193.
Giri, J., et al. (2020). CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Reports, 30(6), 1923–1934 e4.
McDonald, B., et al. (2010). Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science, 330(6002), 362–366.
Mahmoudi, M., et al. (2019). Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. International Immunopharmacology, 74, 105689.
Kwon, M.-Y., et al. (2020). Expression of stromal cell-derived factor-1 by mesenchymal stromal cells impacts neutrophil function during sepsis. Critical Care Medicine, 48(5), e409.
Steinman, R. M., Hawiger, D., & Nussenzweig, M. C. (2003). Tolerogenic dendritic cells. Annual Review of Immunology, 21(1), 685–711.
Yuan, X., et al. (2019). Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nature Communications, 10(1), 2498.
Aldinucci, A., et al. (2010). Inhibition of immune synapse by altered dendritic cell actin distribution: A new pathway of mesenchymal stem cell immune regulation. Journal of Immunology, 185(9), 5102–5110.
Agaugué, S., et al. (2008). Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood, 112(5), 1776–1783.
Moretta, L., et al. (2006). Effector and regulatory events during natural killer–dendritic cell interactions. Immunological Reviews, 214(1), 219–228.
Reinders, M. E., & Hoogduijn, M. J. (2014). NK cells and MSCs: Possible implications for MSC therapy in renal transplantation. Stem Cell Research & Therapy, 4(2), 1000166.
Schraufstatter, I. U., Khaldoyanidi, S. K., & DiScipio, R. G. (2015). Complement activation in the context of stem cells and tissue repair. World Journal of Stem Cells, 7(8), 1090–1108.
Soland, M. A., et al. (2013). Mesenchymal stem cells engineered to inhibit complement-mediated damage. PLoS One, 8(3), e60461.
Salvadori, M., & Bertoni, E. (2016). Complement related kidney diseases: Recurrence after transplantation. World Journal of Transplantation, 6(4), 632–645.
Sato, N., et al. (2011). Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus, 20(13), 1378–1386.
Teixeira, J. E., et al. (1996). CR1 stump peptide and terminal complement complexes are found in the glomeruli of lupus nephritis patients. Clinical and Experimental Immunology, 105(3), 497–503.
Arriens, C., et al. (2017). Systemic lupus erythematosus biomarkers: The challenging quest. Rheumatology (Oxford), 56(suppl_1), i32–i45.
Tan, Y., et al. (2013). Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritis. BMC Nephrology, 14, 63.
Botto, M., et al. (1998). Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genetics, 19(1), 56–59.
Tu, Z., et al. (2010). Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells and Development, 19(11), 1803–1809.
Ma, H., et al. (2018). Mesenchymal stem cells control complement C5 activation by factor H in lupus nephritis. EBioMedicine, 32, 21–30.
Moll, G., et al. (2011). Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS One, 6(7), e21703.
Wang, F. M., et al. (2016). The dysfunctions of complement factor H in lupus nephritis. Lupus, 25(12), 1328–1340.
Stegert, M., Bock, M., & Trendelenburg, M. (2015). Clinical presentation of human C1q deficiency: How much of a lupus? Molecular Immunology, 67(1), 3–11.
Gavin, C., et al. (2019). The complement system is essential for the phagocytosis of mesenchymal stromal cells by monocytes. Frontiers in Immunology, 10, 2249.
Liang, J., & Sun, L. (2015). Mesenchymal stem cells transplantation for systemic lupus erythematosus. International Journal of Rheumatic Diseases, 18(2), 164–171.
Zhao, C., et al. (2019). Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Design, Development and Therapy, 13, 3693–3704.
Nisihara, R. M., et al. (2013). Deposition of the lectin pathway of complement in renal biopsies of lupus nephritis patients. Human Immunology, 74(8), 907–910.
Sadanaga, A., et al. (2007). Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis and Rheumatism, 56(5), 1618–1628.
Ma, X., et al. (2013). Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplantation, 22(12), 2279–2290.
Deng, W., et al. (2005). Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA and Cell Biology, 24(7), 458–463.
Wang, D., et al. (2013). Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplantation, 22(12), 2267–2277.
Yang, X., et al. (2018). Bone marrow-derived mesenchymal stem cells inhibit T follicular helper cell in lupus-prone mice. Lupus, 27(1), 49–59.
Choi, E. W., et al. (2012). Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue–derived mesenchymal stem cell transplantation. Arthritis and Rheumatism, 64(1), 243–253.
Park, M.-J., et al. (2015). Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplantation, 24(11), 2367–2377.
Choi, E. W., et al. (2015). Transplantation of adipose tissue-derived mesenchymal stem cells prevents the development of lupus dermatitis. Stem Cells and Development, 24(17), 2041–2051.
Choi, E. W., et al. (2016). Mesenchymal stem cell transplantation can restore lupus disease-associated miRNA expression and Th1/Th2 ratios in a murine model of SLE. Scientific Reports, 6(1), 1–14.
Zhang, Z., et al. (2019). Mesenchymal stem cells prevent podocyte injury in lupus-prone B6. MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrology, Dialysis, Transplantation, 34(4), 597–605.
Chang, J.-W., et al. (2011). Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplantation, 20(2), 245–258.
Gu, Z., et al. (2010). Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus, 19(13), 1502–1514.
Wang, D., et al. (2018). A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Reports, 10(3), 933–941.
Gu, F., et al. (2014). Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clinical Rheumatology, 33(11), 1611–1619.
Shi, D., et al. (2012). Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clinical Rheumatology, 31(5), 841–846.
Liang, J., et al. (2010). Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Annals of the Rheumatic Diseases, 69(8), 1423–1429.
Sun, L., et al. (2009). Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells, 27(6), 1421–1432.
Yuan, X., et al. (2019). Mesenchymal stem cell therapy induces FLT3L and CD1c+ dendritic cells in systemic lupus erythematosus patients. Nature Communications, 10(1), 1–12.
Sun, L., et al. (2010). Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis and Rheumatism, 62(8), 2467–2475.
Dema, B., et al. (2017). Basophils contribute to pristane-induced lupus-like nephritis model. Scientific Reports, 7(1), 7969.
Wang, Q., et al. (2015). Combined transplantation of autologous hematopoietic stem cells and allogenic mesenchymal stem cells increases T regulatory cells in systemic lupus erythematosus with refractory lupus nephritis and leukopenia. Lupus, 24(11), 1221–1226.
Rezaieyazdi, Z., et al. (2014). Efficacy of long-term maintenance therapy with mycophenolate mofetil in lupus nephritis. Springerplus, 3, 638.
Dang, J., et al. (2020). Human gingiva-derived mesenchymal stem cells are therapeutic in lupus nephritis through targeting of CD39−CD73 signaling pathway. Journal of Autoimmunity, 113, 102491.
Wang, D., et al. (2014). Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Research & Therapy, 16(2), R79.
Schena, F., et al. (2010). Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis and Rheumatism, 62(9), 2776–2786.
Chen, X., Armstrong, M. A., & Li, G. (2006). Mesenchymal stem cells in immunoregulation. Immunology and Cell Biology, 84(5), 413–421.
Fathollahi, A., Gabalou, N. B., & Aslani, S. (2018). Mesenchymal stem cell transplantation in systemic lupus erythematous, a mesenchymal stem cell disorder. Lupus, 27(7), 1053–1064.
Sanz-Nogués, C., & O'Brien, T. (2021). Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomaterials and Biosystems, 2, 100018.
Phinney, D. G., & Galipeau, J. (2019). Manufacturing mesenchymal stromal cells for clinical applications: A survey of good manufacturing practices at US academic centers. Cytotherapy, 21(7), 782–792.
Mizukami, A., et al. (2018). Technologies for large-scale umbilical cord-derived MSC expansion: Experimental performance and cost of goods analysis. Biochemical Engineering Journal, 135, 36–48.
Kelly, K. (2015). Limitless starting materials for large-scale manufacture of MSCs–what does the future hold? Pharmaceutical Bioprocessing, 3(4), 281–283.
Author information
Authors and Affiliations
Contributions
HA has drafted the work. [First Author].
MM, RZ, TAJ thoroughly reviewed it.
MA and HS have drawn the figures.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
“Not applicable”.
Consent for Publication
“Not applicable”.
Competing Interests
All authors contributed equally.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Tissue-resident stem/progenitor cells endowed with broader germ layer specification potential in normal and cancerous tissues
Guest Editor: Deepa Bhartiya
Rights and permissions
About this article
Cite this article
Mahmoudi, M., Hoseinzadeh, A., Rezaieyazdi, Z. et al. Cross Talk between Mesenchymal Stem/Stromal Cells and Innate Immunocytes Concerning Lupus Disease. Stem Cell Rev and Rep 18, 2781–2796 (2022). https://doi.org/10.1007/s12015-022-10397-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10397-x