Abstract
Background
Human induced pluripotent stem cells (hiPSCs) hold great potentials in disease modeling, drug screening and cell therapy. However, efficiency and costs of hiPSCs preparation still need to be improved.
Methods
We screened the compounds that target signaling pathways, epigenetic modifications or metabolic-process regulation to replace the growth factors. After small molecule treatment, TRA-1-60, which is a cell surface antigen expressed by human embryonic stem cells (hESCs), staining was performed to quantify the efficiency of somatic cell reprogramming. Next, small molecule cocktail-induced ESCs or iPSCs were examined with pluripotent markers expression. Finally, Genome-wide gene expression profile was analyzed by RNA-seq to illustrate the mechanism of human somatic cell reprogramming.
Result
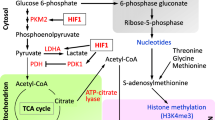
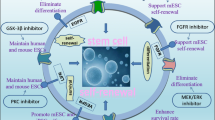
Here, we found that a dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) inhibitor ID-8 robustly enhanced human somatic cell reprogramming by upregulation of pyruvate dehydrogenase kinase 4 (PDK4) and activation of glycolysis. Furthermore, we identified a novel growth-factor-free hiPSC generation system using small molecules ID-8 (I) and TGFβ signal pathway agonist Kartogenin (K). Importantly, we developed IK medium combined with low-dose bFGF to support the long-term expansion of human pluripotent stem cells. IK-iPSCs showed pluripotency and normal karyotype.
Conclusions
Our studies may provide a novel growth-factor-free culture system to facilitate the generation of hiPSCs for multiple applications in regenerative medicine.
Graphical abstract
In Brief
Xu et at. found that a dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) inhibitor ID-8 robustly enhanced human somatic cell reprogramming by upregulation of PDK4 and activation of glycolysis. Furthermore, we established a novel growth-factor-free hiPSC generation system using small molecules ID-8/Kartogenin (IK). IK medium combined with Low-dose bFGF (IKB medium) supported the long-term expansion of human pluripotent stem cells.
Highlights
ID-8 Enhanced Reprogramming of Human Fibroblasts and Astrocytes
Establishment of the Growth-factor-free Reprogramming System Using Small Molecule Compounds IK
IKB Medium Maintained the Long-term Expansion of Human Pluripotent Stem Cells
ID-8 Promoted Human Somatic Cell Reprogramming by Activating PDK4 Expression







Similar content being viewed by others
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- hiPSCs:
-
Human induced pluripotent stem cells
- IKB:
-
ID-8, Kartogenin and Low-dose bFGF
- E8:
-
Essential 8
- E6:
-
E8 without bFGF and TGFβ
- I:
-
ID-8
- K:
-
Kartogenin
- C:
-
CHIR99021
- AK:
-
1-Azakenpaullone
- T:
-
Tacrolimus
- IK-iPSCs:
-
iPSC cultured in E6 with ID-8 and Kartogenin
- TZDs:
-
Thiazolidinediones
- DCA:
-
Sodium dichloroacetate
- 2-DG:
-
2-Deoxyglucose
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- Go:
-
Go6983
- SP:
-
SP600125
- Dora:
-
Doramapimod
- DGs:
-
Differential genes
References
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Yu, J. Y., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920.
Robinton, D. A., & Daley, G. Q. (2012). The promise of induced pluripotent stem cells in research and therapy. Nature, 481, 295–305.
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy- promise and challenges. Cell Stem Cell, 27, 523–531.
Haridhasapavalan, K. K., et al. (2019). An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene, 686, 146–159.
Hu, K. J. (2014). All roads lead to induced pluripotent stem cells: The technologies of iPSC generation. Stem Cells Dev, 23, 1285–1300.
Jia, F., et al. (2010). A nonviral minicircle vector for deriving human iPS cells. Nature methods, 7, 197–199.
Okita, K., et al. (2011). A more efficient method to generate integration-free human iPS cells. Nature methods, 8, 409–412.
Yasuda, S. Y., et al. (2018). Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nature Biomedical Engineering, 2, 173–182.
Bar-Nur, O., et al. (2014). Small molecules facilitate rapid and synchronous iPSC generation. Nature Methods, 11, 1170–1176.
Jung, D. W., Kim, W. H., & Williams, D. R. (2014). Reprogram or reboot: Small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chemical Biology, 9, 80–95.
Shao, Z., et al. (2016). Reprogramming by De-bookmarking the somatic transcriptional program through targeting of BET bromodomains. Cell Reports, 16, 3138–3145.
Chen, G., Guo, Y., Li, C., Li, S., & Wan, X. (2020). Small molecules that promote self-renewal of stem cells and somatic cell reprogramming. Stem Cell Reviews and Reports, 16, 511–523.
Huangfu, D., et al. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnology, 26, 795–797.
Shi, Y., et al. (2008). Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell, 3, 568–574.
Ichida, J. K., et al. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5, 491–503.
Zhu, S., et al. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell, 7, 651–655.
Plath, K., & Lowry, W. E. (2011). Progress in understanding reprogramming to the induced pluripotent state. Nature Reviews Genetics, 12, 253–265.
Takahashi, K., & Yamanaka, S. (2016). A decade of transcription factor-mediated reprogramming to pluripotency. Nature Reviews Molecular Cell Biology, 17, 183–193.
Liu, G., David, B. T., Trawczynski, M., & Fessler, R. G. (2020). Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Reviews and Reports, 16, 3–32.
de I’Hortet, A. C., et al. (2019). Generation of human fatty livers using custom-engineered induced pluripotent stem cells with modifiable SIRT1 metabolism. Cell Metabolism, 30, 385–401.
Ouchi, R., et al. (2019). Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metabolism, 30, 374–386.
Doi, D., et al. (2020). Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nature Communications, 11.
Xu, Z., et al. (2016). Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc Natl Acad Sci U S A, 113, 6382–6390.
Hasegawa, K., et al. (2012). Wnt signaling orchestration with a small molecule DYRK inhibitor provides long-term xeno-free human pluripotent cell expansion. Stem Cells Translational Medicine, 1, 18–28.
Beers, J., et al. (2012). Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nature Protocols, 7, 2029–2040.
Chen, G. K., et al. (2011). Chemically defined conditions for human iPSC derivation and culture. Nature Methods, 8, 424–476.
Greber, B., Lehrach, H., & Adjaye, J. (2007). Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells, 25, 455–464.
Abbot, E. L., et al. (2005). Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells. FEBS Journal, 272, 3004–3014.
Cadoudal, T., et al. (2008). Pyruvate dehydrogenase kinase 4 - Regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes, 57, 2272–2279.
Stoukides, D. M., Jacobson, E. F., & Tzanakakis, E. S. (2021). Scalable expansion of human pluripotent stem cells for biomanufacturing cellular therapeutics. In Methods in iPSC Technology (pp. 289–308).
Lai, K. K. Y., & Kahn, M. (2021). Pharmacologically targeting the WNT/beta-Catenin signaling cascade: Avoiding the Sword of damocles. Handb Exp Pharmacol.
Fei, T., & Chen, Y. G. (2010). Regulation of embryonic stem cell self-renewal and differentiation by TGF-beta family signaling. Sci China Life Sci, 53, 497–503.
Li, W., Wei, W., & Ding, S. (2016). TGF-beta signaling in stem cell regulation. Methods in Molecular Biology, 1344, 137–145.
Wang, J., Zhou, J., Zhang, N., Zhang, X. L., & Li, Q. F. (2014). A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Bioph Res Co, 450, 568–574.
Kuo, H. H., et al. (2020). Negligible-cost and weekend-free chemically defined human iPSC culture. Stem Cell Reports, 14, 256–270.
Folmes, C. D. L., et al. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabolism, 14, 264–271.
Ryall, J. G., Cliff, T., Dalton, S., & Sartorelli, V. (2015). Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell, 17, 651–662.
Nishimura, K., Fukuda, A., & Hisatake, K. (2019). Mechanisms of the metabolic shift during somatic cell reprogramming. Int J Mol Sci, 20.
Ishida, T., Nakao, S., Ueyama, T., Harada, Y., & Kawamura, T. (2020). Metabolic remodeling during somatic cell reprogramming to induced pluripotent stem cells: involvement of hypoxia-inducible factor 1. Inflamm Regen, 40.
Prigione, A., et al. (2014). HIF1 alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells, 32, 364–376.
Han, J. E., et al. (2017). Inhibition of HIF1 alpha and PDK induces cell death of glioblastoma multiforme. Exp Neurobiol, 26, 295–306.
Sradhanjali, S., & Reddy, M. M. (2018). Inhibition of pyruvate dehydrogenase kinase as a therapeutic strategy against cancer. Current Topics in Medicinal Chemistry, 18, 444–453.
Acknowledgements
We would like to thank School of Medicine, Xiamen University and MOE Frontiers Center for Brain Science, Fudan University for providing platform for experiments of cell culture, staining and images.
Funding
This work was supported by National Natural Science Foundation of China (grants 32070956 to Zhicheng Shao), Fundamental Research Funds for Central Universities from Xiamen Universities (grants 20720180040 to Zhicheng Shao), Natural Science Foundation of Shanghai (grants 20ZR1405200 to Zhicheng Shao), Natural Science Foundation of Fujian Province of China (grants 2018J01055 to Zhicheng Shao), MOE Frontiers Center for Brain Science fund and starting fund from Fudan Universities.
Author information
Authors and Affiliations
Contributions
Z.S and J.X. designed the experiments. J.X., S.F., N.W., B.L., Y.H., Q.F., J.S.and H.L. performed fibroblast and astrocyte reprogramming, screening of small molecules for promoting reprogramming efficiency and maintaining human pluripotency stem cells. Z.S and J.X. did RNA-seq analysis. Z.S., J.X. and N.W. wrote the manuscript and data interpretation. Z.S. supported this study financially.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Stem Cell Technology and Skin Disorders (Dermatology): from Stem Cell Biology to Clinical Application
Guest Editor: Ali Golchin
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Fang, S., Wang, N. et al. Dual-specificity Tyrosine Phosphorylation-regulated Kinase Inhibitor ID-8 Promotes Human Somatic Cell Reprogramming by Activating PDK4 Expression. Stem Cell Rev and Rep 18, 2074–2087 (2022). https://doi.org/10.1007/s12015-021-10294-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10294-9