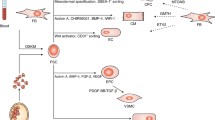
Abstract
The induced pluripotent stem cells (iPSCs) are derived from somatic cells by using reprogramming factors such as Oct4, Sox2, Klf4, and c-Myc (OSKM) or Oct4, Sox2, Nanog and Lin28 (OSNL). They resemble embryonic stem cells (ESCs) and have the ability to differentiate into cell lineage of all three germ-layer, including cardiomyocytes (CMs). The CMs can be generated from iPSCs by inducing embryoid bodies (EBs) formation and treatment with activin A, bone morphogenic protein 4 (BMP4), and inhibitors of Wnt signaling. However, these iPSC-derived CMs are a heterogeneous population of cells and require purification and maturation to mimic the in vivo CMs. The matured CMs can be used for various therapeutic purposes in regenerative medicine by cardiomyoplasty or through the development of tissue-engineered cardiac patches. In recent years, significant advancements have been made in the isolation of iPSC and their differentiation, purification, and maturation into clinically usable CMs. Newer small molecules have also been identified to substitute the reprogramming factors for iPSC generation as well as for direct differentiation of somatic cells into CMs without an intermediary pluripotent state. This review provides a concise update on the generation of iPSC-derived CMs and their application in personalized cardiac regenerative medicine. It also discusses the current limitations and challenges in the application of iPSC-derived CMs.

Graphical abstract




Similar content being viewed by others
References
Narazaki, G., Uosaki, H., Teranishi, M., Okita, K., Kim, B., Matsuoka, S., Yamanaka, S., & Yamashita, J. K. (2008). Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation, 118, 498–506.
Zhang, J., Wilson, G. F., Soerens, A. G., Koonce, C. H., Yu, J., Palecek, S. P., Thomson, J. A., & Kamp, T. J. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research, 104, e30–e41.
Mauritz, C., Schwanke, K., Reppel, M., Neef, S., Katsirntaki, K., Maier, L. S., Nguemo, F., Menke, S., Haustein, M., Hescheler, J., Hasenfuss, G., & Martin, U. (2008). Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation, 118, 507–517.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., & Thomson, J. A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920.
Moore, J. C. (2013). Generation of human-induced pluripotent stem cells by lentiviral transduction. Methods in Molecular Biology, 997, 35–43.
Zhou, T., Benda, C., Duzinger, S., Huang, Y., Li, X., Li, Y., Guo, X., Cao, G., Chen, S., Hao, L., Chan, Y. C., Ng, K. M., Ho, J. C., Wieser, M., Wu, J., Redl, H., Tse, H. F., Grillari, J., Grillari-Voglauer, R., Pei, D., & Esteban, M. A. (2011). Generation of induced pluripotent stem cells from urine. American Society of Nephrology, 22, 1221–1228.
Macarthur, C. C., Fontes, A., Ravinder, N., Kuninger, D., Kaur, J., Bailey, M., Taliana, A., Vemuri, M. C., & Lieu, P. T. (2012). Generation of human-induced pluripotent stem cells by a nonintegrating RNA Sendai virus vector in feeder-free or xeno-free conditions. Stem Cells International, 2012, 564612.
Yu, J., Hu, K., Smuga-Otto, K., Tian, S., Stewart, R., Slukvin, I. I., & Thomson, J. A. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science, 324, 797–801.
Kim, D., Kim, C. H., Moon, J. I., Chung, Y. G., Chang, M. Y., Han, B. S., Ko, S., Yang, E., Cha, K. Y., Lanza, R., & Kim, K. S. (2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell, 4, 472–476.
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y. H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A., Daley, G. Q., Brack, A. S., Collins, J. J., Cowan, C., Schlaeger, T. M., & Rossi, D. J. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 7, 618–630.
Steinle, H., Weber, M., Behring, A., Mau-Holzmann, U., von Ohle, C., Popov, A. F., Schlensak, C., Wendel, H. P., & Avci-Adali, M. (2019). Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Molecular Therapy--Nucleic Acids, 17, 907–921.
Sandmaier, S. E., & Telugu, B. P. (2015). MicroRNA-Mediated Reprogramming of Somatic Cells into Induced Pluripotent Stem Cells. Methods in Molecular Biology, 1330, 29–36.
Woltjen, K., Michael, I. P., Mohseni, P., Desai, R., Mileikovsky, M., Hämäläinen, R., Cowling, R., Wang, W., Liu, P., Gertsenstein, M., Kaji, K., Sung, H. K., & Nagy, A. (2009). piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature, 458, 766–770.
Melo, U. S., de Souza Leite, F., Costa, S., Rosenberg, C., & Zatz, M. (2018). A fast method to reprogram and CRISPR/Cas9 gene editing from erythroblasts. Stem Cell Research, 31, 52–54.
Weltner, J., Balboa, D., Katayama, S., Bespalov, M., Krjutskov, K., Jouhilahti, E. M., Trokovic, R., Kere, J., & Otonkoski, T. (2018). Human pluripotent reprogramming with CRISPR activators. Nature Communications, 9, 2643.
Chakraborty, S., Christoforou, N., Fattahi, A., Herzog, R. W., & Leong, K. W. (2013). A robust strategy for negative selection of Cre-loxP recombination-based excision of transgenes in induced pluripotent stem cells. PLoS One, 8, e64342.
Kadari, A., Lu, M., Li, M., Sekaran, T., Thummer, R. P., Guyette, N., Chu, V., & Edenhofer, F. (2014). Excision of viral reprogramming cassettes by Cre protein transduction enables rapid, robust and efficient derivation of transgene-free human induced pluripotent stem cells. Stem Cell Research & Therapy, 5, 47.
Morishige, S., Mizuno, S., Ozawa, H., Nakamura, T., Mazahery, A., Nomura, K., Seki, R., Mouri, F., Osaki, K., Yamamura, K., Okamura, T., & Nagafuji, K. (2020). CRISPR/Cas9-mediated gene correction in hemophilia B patient-derived iPSCs. International Journal of Hematology, 111, 225–233.
Ma, J., Zhang, J., He, J., Zhang, Z., Li, W., Feng, B., Guo, R., Amponsah, A. E., Kong, D., Liu, A., Song, Y., Wei, L., & Cui, H. (2020). Induced pluripotent stem cell (iPSC) line (HEBHMUi002-A) from a healthy female individual and neural differentiation. Stem Cell Research, 42, 101669.
Lee, C. H., Ingrole, R. S. J., & Gill, H. S. (2020). Generation of induced pluripotent stem cells using elastin like polypeptides as a non-viral gene delivery system. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1866, 165405.
Lanzi, G., Ferraro, R. M., Masneri, S., Piovani, G., Barisani, C., Sobacchi, C., Villa, A., Vezzoni, P., & Giliani, S. (2020). Generation of 3 clones of induced pluripotent stem cells (iPSCs) from a patient affected by Autosomal Recessive Osteopetrosis due to mutations in TCIRG1 gene. Stem Cell Research, 42, 101660.
Woods, S., Bates, N., Dunn, S. L., Serracino-Inglott, F., Hardingham, T. E., & Kimber, S. J. (2020). Generation of Human-Induced Pluripotent Stem Cells From Anterior Cruciate Ligament. Journal of Orthopaedic Research, 38, 92–104.
Horton, C., Davies, T. J., Lahiri, P., Sachamitr, P., & Fairchild, P. J. (2020). Induced pluripotent stem cells reprogrammed from primary dendritic cells provide an abundant source of immunostimulatory dendritic cells for use in immunotherapy. Stem Cells, 38, 67–79.
Mulder, J., Sharmin, S., Chow, T., Rodrigues, D. C., Hildebrandt, M. R., D'Cruz, R., Rogers, I., Ellis, J., & Rosenblum, N. D. (2020). Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatric Research, 87, 647–655.
Hiramoto, T., Tahara, M., Liao, J., Soda, Y., Miura, Y., Kurita, R., Hamana, H., Inoue, K., Kohara, H., Miyamoto, S., Hijikata, Y., Okano, S., Yamaguchi, Y., Oda, Y., Ichiyanagi, K., Toh, H., Sasaki, H., Kishi, H., Ryo, A., Muraguchi, A., Takeda, M., & Tani, K. (2020). Non-transmissible MV Vector with Segmented RNA Genome Establishes Different Types of iPSCs from Hematopoietic Cells. Molecular Therapy, 28, 129–141.
Sung, T. C., Li, H. F., Higuchi, A., Kumar, S. S., Ling, Q. D., Wu, Y. W., Burnouf, T., Nasu, M., Umezawa, A., Lee, K. F., Wang, H. C., Chang, Y., & Hsu, S. T. (2020). Effect of cell culture biomaterials for completely xeno-free generation of human induced pluripotent stem cells. Biomaterials, 230, 119638.
Ustyantseva, E. I., Medvedev, S. P., Vetchinova, A. S., Illarioshkin, S. N., Leonov, S. V., & Zakian, S. M. (2020). Generation of an induced pluripotent stem cell line, ICGi014-A, by reprogramming peripheral blood mononuclear cells from a patient with homozygous D90A mutation in SOD1 causing Amyotrophic lateral sclerosis. Stem Cell Research, 42, 101675.
Trionfini, P., Ciampi, O., Romano, E., Benigni, A., & Tomasoni, S. (2020). Generation of two isogenic knockout PKD2 iPS cell lines, IRFMNi003-A-1 and IRFMNi003-A-2, using CRISPR/Cas9 technology. Stem Cell Research, 42, 101667.
Yang, T., Qin, J., Zhang, Q., Sun, H., Wang, Z., Yang, J., Liu, H., Zhang, C., Zhang, S., Zhang, J., Wang, Y., & Xu, Y. (2020). Generation of induced pluripotent stem cell line (ZZUi0018-A ) from a patient with spinocerebellar ataxia type 6. Stem Cell Research, 44, 101777.
Lu, H. E., Tsai, C. L., Chiu, I. M., Pan, Y. L., Lin, Y. F., Lin, H. C., & Hsu, Y. C. (2020). Generation of induced pluripotent stem cells MMCi001-A from a Taiwanese hearing loss patient carrying GJB2 pV37I mutation. Stem Cell Research, 42, 101692.
Slamecka, J., Salimova, L., McClellan, S., van Kelle, M., Kehl, D., Laurini, J., Cinelli, P., Owen, L., Hoerstrup, S. P., & Weber, B. (2016). Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions. Cell Cycle, 15, 234–249.
Ye, J., Ge, J., Zhang, X., Cheng, L., Zhang, Z., He, S., Wang, Y., Lin, H., Yang, W., Liu, J., Zhao, Y., & Deng, H. (2016). Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Research, 26, 34–45.
Li, D., Wang, L., Hou, J., Shen, Q., Chen, Q., Wang, X., Du, J., Cai, X., Shan, Y., Zhang, T., Zhou, T., Shi, X., Li, Y., Zhang, H., & Pan, G. (2016). Optimized Approaches for Generation of Integration-free iPSCs from Human Urine-Derived Cells with Small Molecules and Autologous Feeder. Stem Cell Reports, 6, 717–728.
Zhao, Y., Zhao, T., Guan, J., Zhang, X., Fu, Y., Ye, J., Zhu, J., Meng, G., Ge, J., Yang, S., Cheng, L., Du, Y., Zhao, C., Wang, T., Su, L., Yang, W., & Deng, H. (2015). A XEN-like State Bridges Somatic Cells to Pluripotency during Chemical Reprogramming. Cell, 163, 1678–1691.
Rais, Y., Zviran, A., Geula, S., Gafni, O., Chomsky, E., Viukov, S., Mansour, A. A., Caspi, I., Krupalnik, V., Zerbib, M., Maza, I., Mor, N., Baran, D., Weinberger, L., Jaitin, D. A., Lara-Astiaso, D., Blecher-Gonen, R., Shipony, Z., Mukamel, Z., Hagai, T., Gilad, S., Amann-Zalcenstein, D., Tanay, A., Amit, I., Novershtern, N., & Hanna, J. H. (2013). Deterministic direct reprogramming of somatic cells to pluripotency. Nature, 502, 65–70.
Hou, P., Li, Y., Zhang, X., Liu, C., Guan, J., Li, H., Zhao, T., Ye, J., Yang, W., Liu, K., Ge, J., Xu, J., Zhang, Q., Zhao, Y., & Deng, H. (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 341, 651–654.
Thier, M., Munst, B., Mielke, S., & Edenhofer, F. (2012). Cellular reprogramming employing recombinant sox2 protein. Stem Cells International, 2012, 549846.
Li, Y., Zhang, Q., Yin, X., Yang, W., Du, Y., Hou, P., Ge, J., Liu, C., Zhang, W., Zhang, X., Wu, Y., Li, H., Liu, K., Wu, C., Song, Z., Zhao, Y., Shi, Y., & Deng, H. (2011). Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Research, 21, 196–204.
Anokye-Danso, F., Trivedi, C. M., Juhr, D., Gupta, M., Cui, Z., Tian, Y., Zhang, Y., Yang, W., Gruber, P. J., Epstein, J. A., & Morrisey, E. E. (2011). Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell, 8, 376–388.
Yu, J., Chau, K. F., Vodyanik, M. A., Jiang, J., & Jiang, Y. (2011). Efficient feeder-free episomal reprogramming with small molecules. PLoS One, 6, e17557.
Staerk, J., Lyssiotis, C. A., Medeiro, L. A., Bollong, M., Foreman, R. K., Zhu, S., Garcia, M., Gao, Q., Bouchez, L. C., Lairson, L. L., Charette, B. D., Supekova, L., Janes, J., Brinker, A., Cho, C. Y., Jaenisch, R., & Schultz, P. G. (2011). Pan-Src family kinase inhibitors replace Sox2 during the direct reprogramming of somatic cells. Angewandte Chemie (International Ed. in English), 50, 5734–5736.
Si-Tayeb, K., Noto, F. K., Sepac, A., Sedlic, F., Bosnjak, Z. J., Lough, J. W., & Duncan, S. A. (2010). Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Developmental Biology, 10, 81.
Chan, A. W., Cheng, P. H., Neumann, A., & Yang, J. J. (2010). Reprogramming Huntington monkey skin cells into pluripotent stem cells. Cellular Reprogramming, 12, 509–517.
Cho, H. J., Lee, C. S., Kwon, Y. W., Paek, J. S., Lee, S. H., Hur, J., Lee, E. J., Roh, T. Y., Chu, I. S., Leem, S. H., Kim, Y., Kang, H. J., Park, Y. B., & Kim, H. S. (2010). Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood, 116, 386–395.
Heng, J. C., Feng, B., Han, J., Jiang, J., Kraus, P., Ng, J. H., Orlov, Y. L., Huss, M., Yang, L., Lufkin, T., Lim, B., & Ng, H. H. (2010). The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell, 6, 167–174.
Haase, A., Olmer, R., Schwanke, K., Wunderlich, S., Merkert, S., Hess, C., Zweigerdt, R., Gruh, I., Meyer, J., Wagner, S., Maier, L. S., Han, D. W., Glage, S., Miller, K., Fischer, P., Scholer, H. R., & Martin, U. (2009). Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell, 5, 434–441.
Ye, Z., Zhan, H., Mali, P., Dowey, S., Williams, D. M., Jang, Y. Y., Dang, C. V., Spivak, J. L., Moliterno, A. R., & Cheng, L. (2009). Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood, 114, 5473–5480.
Lyssiotis, C. A., Foreman, R. K., Staerk, J., Garcia, M., Mathur, D., Markoulaki, S., Hanna, J., Lairson, L. L., Charette, B. D., Bouchez, L. C., Bollong, M., Kunick, C., Brinker, A., Cho, C. Y., Schultz, P. G., & Jaenisch, R. (2009). Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proceedings of the National Academy of Sciences of the United States of America, 106, 8912–8917.
Feng, B., Jiang, J., Kraus, P., Ng, J. H., Heng, J. C., Chan, Y. S., Yaw, L. P., Zhang, W., Loh, Y. H., Han, J., Vega, V. B., Cacheux-Rataboul, V., Lim, B., Lufkin, T., & Ng, H. H. (2009). Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nature Cell Biology, 11, 197–203.
Stadtfeld, M., Brennand, K., & Hochedlinger, K. (2008). Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Current Biology, 18, 890–894.
Nakagawa, M., Koyanagi, M., Tanabe, K., Takahashi, K., Ichisaka, T., Aoi, T., Okita, K., Mochiduki, Y., Takizawa, N., & Yamanaka, S. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology, 26, 101–106.
Shi, Y., Do, J. T., Desponts, C., Hahm, H. S., Schöler, H. R., & Ding, S. (2008). A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell, 2, 525–528.
Wernig, M., Meissner, A., Foreman, R., Brambrink, T., Ku, M., Hochedlinger, K., Bernstein, B. E., & Jaenisch, R. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448, 318–324.
Stadtfeld, M., Apostolou, E., Akutsu, H., Fukuda, A., Follett, P., Natesan, S., Kono, T., Shioda, T., & Hochedlinger, K. (2010). Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature, 465, 175–181.
Stadtfeld, M., & Hochedlinger, K. (2010). Induced pluripotency: history, mechanisms, and applications. Genes & Development, 24, 2239–2263.
Maekawa, M., Yamaguchi, K., Nakamura, T., Shibukawa, R., Kodanaka, I., Ichisaka, T., Kawamura, Y., Mochizuki, H., Goshima, N., & Yamanaka, S. (2011). Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature, 474, 225–229.
Zhao, Y., Yin, X., Qin, H., Zhu, F., Liu, H., Yang, W., Zhang, Q., Xiang, C., Hou, P., Song, Z., Liu, Y., Yong, J., Zhang, P., Cai, J., Liu, M., Li, H., Li, Y., Qu, X., Cui, K., Zhang, W., Xiang, T., Wu, Y., Liu, C., Yu, C., Yuan, K., Lou, J., Ding, M., & Deng, H. (2008). Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell, 3, 475–479.
Chen, K., Long, Q., Xing, G., Wang, T., Wu, Y., Li, L., Qi, J., Zhou, Y., Ma, B., Schöler, H. R., Nie, J., Pei, D., & Liu, X. (2020). Heterochromatin loosening by the Oct4 linker region facilitates Klf4 binding and iPSC reprogramming. The EMBO Journal, 39, e99165.
Seki, T., Yuasa, S., Oda, M., Egashira, T., Yae, K., Kusumoto, D., Nakata, H., Tohyama, S., Hashimoto, H., Kodaira, M., Okada, Y., Seimiya, H., Fusaki, N., Hasegawa, M., & Fukuda, K. (2010). Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell, 7, 11–14.
Akkouh, I. A., Ueland, T., Hansson, L., Inderhaug, E., Hughes, T., Steen, N. E., Aukrust, P., Andreassen, O. A., Szabo, A., & Djurovic, S. (2020). Decreased IL-1β-induced CCL20 response in human iPSC-astrocytes in schizophrenia: Potential attenuating effects on recruitment of regulatory T cells. Brain, Behavior, and Immunity, 87, 634–644.
Alari, V., Russo, S., Rovina, D., Garzo, M., Crippa, M., Calzari, L., Scalera, C., Concolino, D., Castiglioni, E., Giardino, D., Prosperi, E., Finelli, P., Gervasini, C., Gowran, A., & Larizza, L. (2019). Generation of three iPSC lines (IAIi002, IAIi004, IAIi003) from Rubinstein-Taybi syndrome 1 patients carrying CREBBP non sense c.4435G>T, p.(Gly1479*) and c.3474G>A, p.(Trp1158*) and missense c.4627G>T, p.(Asp1543Tyr) mutations. Stem Cell Research, 40, 101553.
Altieri, F., D'Anzi, A., Martello, F., Tardivo, S., Spasari, I., Ferrari, D., Bernardini, L., Lamorte, G., Mazzoccoli, G., Valente, E. M., Vescovi, A. L., & Rosati, J. (2019). Production and characterization of human induced pluripotent stem cells (iPSC) CSSi007-A (4383) from Joubert Syndrome. Stem Cell Research, 38, 101480.
Bursch, F., Kalmbach, N., Naujock, M., Staege, S., Eggenschwiler, R., Abo-Rady, M., Japtok, J., Guo, W., Hensel, N., Reinhardt, P., Boeckers, T. M., Cantz, T., Sterneckert, J., Van Den Bosch, L., Hermann, A., Petri, S., & Wegner, F. (2019). Altered calcium dynamics and glutamate receptor properties in iPSC-derived motor neurons from ALS patients with C9orf72, FUS, SOD1 or TDP43 mutations. Human Molecular Genetics, 28, 2835–2850.
Anastasaki, C., Wegscheid, M. L., Hartigan, K., Papke, J. B., Kopp, N. D., Chen, J., Cobb, O., Dougherty, J. D., & Gutmann, D. H. (2020). Human iPSC-Derived Neurons and Cerebral Organoids Establish Differential Effects of Germline NF1 Gene Mutations. Stem Cell Reports, 14, 541–550.
Arribas-Carreira, L., Bravo-Alonso, I., López-Márquez, A., Alonso-Barroso, E., Briso-Montiano, Á., Arroyo, I., Ugarte, M., Pérez, B., Pérez-Cerdá, C., Rodríguez-Pombo, P., & Richard, E. (2019). Generation and characterization of a human iPSC line (UAMi005-A) from a patient with nonketotic hyperglycinemia due to mutations in the GLDC gene. Stem Cell Research, 39, 101503.
Atchison, L., Abutaleb, N. O., Snyder-Mounts, E., Gete, Y., Ladha, A., Ribar, T., Cao, K., & Truskey, G. A. (2020). iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome. Stem Cell Reports, 14, 325–337.
Barabino, A., Flamier, A., Hanna, R., Héon, E., Freedman, B. S., & Bernier, G. (2020). Deregulation of Neuro-Developmental Genes and Primary Cilium Cytoskeleton Anomalies in iPSC Retinal Sheets from Human Syndromic Ciliopathies. Stem Cell Reports, 14, 357–373.
Bozaoglu, K., Gao, Y., Stanley, E., Fanjul-Fernández, M., Brown, N. J., Pope, K., Green, C. C., Vlahos, K., Sourris, K., Bahlo, M., Delatycki, M., Scheffer, I., & Lockhart, P. J. (2019). Generation of seven iPSC lines from peripheral blood mononuclear cells suitable to investigate Autism Spectrum Disorder. Stem Cell Research, 39, 101516.
Ding, Y., Marcó de la Cruz, B., Xia, Y., Liu, M., Lu, Y., McInerney, V., Krawczyk, J., Lynch, S. A., Howard, L., O'Brien, T., Gallagher, L., & Shen, S. (2019). Derivation of familial iPSC lines from three ASD patients carrying NRXN1α(+/−) and two controls (NUIGi022-A, NUIGi022-B; NUIGi023-A, NUIGi023-B; NUIGi025-A, NUIGi025-B; NUIGi024-A, NUIGi024-B; NUIGi026-A, NUIGi026-B). Stem Cell Research, 41, 101653.
Bolinches-Amorós, A., León, M., Del Buey Furió, V., Marfany, G., Gonzàlez-Duarte, R., Erceg, S., & Lukovic, D. (2019). Generation of an iPSC line from a retinitis pigmentosa patient carrying a homozygous mutation in CERKL and a healthy sibling. Stem Cell Research, 38, 101455.
Booth, H. D. E., Wessely, F., Connor-Robson, N., Rinaldi, F., Vowles, J., Browne, C., Evetts, S. G., Hu, M. T., Cowley, S. A., Webber, C., & Wade-Martins, R. (2019). RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson's iPSC-derived astrocytes. Neurobiology of Disease, 129, 56–66.
Brazdis, R. M., Alecu, J., Marsch, D., Dahms, A., Simmnacher, K., Lörentz, S., Brendler, A., Schneider, Y., Marxreiter, F., Roybon, L., Winner, B., Xiang, W., & Prots, I. (2020). Demonstration of brain region-specific neuronal vulnerability in human iPSC-based model of familial Parkinson's disease. Human Molecular Genetics, 29, 1180–1191.
Chen, Z., Peng, F., Liu, J., Xie, B., Xu, P., Gan, Z., Li, M., Xu, L., & Zhong, X. (2020). Generation of an iPSC line (SKLOi001-A) from a patient with CLCN2-related leukoencephalopathy. Stem Cell Research, 45, 101769.
Cheng, Y. F., Chan, Y. H., Hu, C. J., Lu, Y. C., Saeki, T., Hosoya, M., Saegusa, C., Fujioka, M., Okano, H., Weng, S. M., Hsu, C. J., Chang, K. H., & Wu, C. C. (2019). Generation of a human iPS cell line (CGMH.SLC26A4919–2) from a Pendred syndrome patient carrying SLC26A4 c.919-2A>G splice-site mutation. Stem Cell Research, 40, 101524.
Erkilic, N., Sanjurjo-Soriano, C., Diakatou, M., Manes, G., Dubois, G., Hamel, C. P., Meunier, I., & Kalatzis, V. (2019). Generation of a human iPSC line, INMi003-A, with a missense mutation in CRX associated with autosomal dominant cone-rod dystrophy. Stem Cell Research, 38, 101478.
Ali, M., Kabir, F., Thomson, J. J., Ma, Y., Qiu, C., Delannoy, M., Khan, S. Y., & Riazuddin, S. A. (2019). Comparative transcriptome analysis of hESC- and iPSC-derived lentoid bodies. Scientific Reports, 9, 18552.
Bueno, C., Sardina, J. L., Di Stefano, B., Romero-Moya, D., Muñoz-López, A., Ariza, L., Chillón, M. C., Balanzategui, A., Castaño, J., Herreros, A., Fraga, M. F., Fernández, A., Granada, I., Quintana-Bustamante, O., Segovia, J. C., Nishimura, K., Ohtaka, M., Nakanishi, M., Graf, T., & Menendez, P. (2016). Reprogramming human B cells into induced pluripotent stem cells and its enhancement by C/EBPα. Leukemia, 30, 674–682.
Rizzi, R., Di Pasquale, E., Portararo, P., Papait, R., Cattaneo, P., Latronico, M. V., Altomare, C., Sala, L., Zaza, A., Hirsch, E., Naldini, L., Condorelli, G., & Bearzi, C. (2012). Post-natal cardiomyocytes can generate iPS cells with an enhanced capacity toward cardiomyogenic re-differentation. Cell Death and Differentiation, 19, 1162–1174.
Cai, W., Zhang, Y., & Kamp, T. J. (2011). Imaging of Induced Pluripotent Stem Cells: From Cellular Reprogramming to Transplantation. American Journal of Nuclear Medicine and Molecular Imaging, 1, 18–28.
Badenes, S. M., Fernandes, T. G., Cordeiro, C. S., Boucher, S., Kuninger, D., Vemuri, M. C., Diogo, M. M., & Cabral, J. M. (2016). Defined Essential 8 Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS One, 11, e0151264.
Badenes, S. M., Fernandes, T. G., Rodrigues, C. A., Diogo, M. M., & Cabral, J. M. (2015). Scalable expansion of human-induced pluripotent stem cells in xeno-free microcarriers. Methods in Molecular Biology, 1283, 23–29.
Rodrigues, A. L., Rodrigues, C. A. V., Gomes, A. R., Vieira, S. F., Badenes, S. M., Diogo, M. M., & Cabral, J. M. S. (2019). Dissolvable Microcarriers Allow Scalable Expansion And Harvesting Of Human Induced Pluripotent Stem Cells Under Xeno-Free Conditions. Biotechnology Journal, 14, e1800461.
Nogueira, D. E. S., Rodrigues, C. A. V., Carvalho, M. S., Miranda, C. C., Hashimura, Y., Jung, S., Lee, B., & Cabral, J. M. S. (2019). Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel bioreactors. Journal of Biological Engineering, 13, 74.
Rodrigues, C. A., Silva, T. P., Nogueira, D. E., Fernandes, T. G., Hashimura, Y., Wesselschmidt, R., Diogo, M. M., Lee, B., & Cabral, J. M. (2018). Scalable culture of human induced pluripotent cells on microcarriers under xeno-free conditions using single-use vertical-wheel™ bioreactors. Journal of Chemical Technology & Biotechnology, 93, 3597–3606.
Abecasis, B., Aguiar, T., Arnault, E., Costa, R., Gomes-Alves, P., Aspegren, A., Serra, M., & Alves, P. M. (2017). Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: Bioprocess intensification and scaling-up approaches. Journal of Biotechnology, 246, 81–93.
Kropp, C., Kempf, H., Halloin, C., Robles-Diaz, D., Franke, A., Scheper, T., Kinast, K., Knorpp, T., Joos, T. O., Haverich, A., Martin, U., Zweigerdt, R., & Olmer, R. (2016). Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Translational Medicine, 5, 1289–1301.
Cabral, J. M. S., & Da Silva, C. L. (2018). Bioreactors for Stem Cell Expansion and Differentiation. Boca Raton: CRC Press.
Lavon, N., Zimerman, M., & Itskovitz-Eldor, J. (2018). Scalable Expansion of Pluripotent Stem Cells. Advances in Biochemical Engineering/Biotechnology, 163, 23–37.
Davis, R. L., Weintraub, H., & Lassar, A. B. (1987). Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell, 51, 987–1000.
Masui, S., Nakatake, Y., Toyooka, Y., Shimosato, D., Yagi, R., Takahashi, K., Okochi, H., Okuda, A., Matoba, R., Sharov, A. A., Ko, M. S., & Niwa, H. (2007). Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nature Cell Biology, 9, 625–635.
Niwa, H., Ogawa, K., Shimosato, D., & Adachi, K. (2009). A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature, 460, 118–122.
Gonzalez, F., Boue, S., & Izpisua Belmonte, J. C. (2011). Methods for making induced pluripotent stem cells: reprogramming a la carte. Nature Reviews. Genetics, 12, 231–242.
Nakagawa, M., Takizawa, N., Narita, M., Ichisaka, T., & Yamanaka, S. (2010). Promotion of direct reprogramming by transformation-deficient Myc. Proceedings of the National Academy of Sciences of the United States of America, 107, 14152–14157.
Mitsui, K., Tokuzawa, Y., Itoh, H., Segawa, K., Murakami, M., Takahashi, K., Maruyama, M., Maeda, M., & Yamanaka, S. (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell, 113, 631–642.
Shyh-Chang, N., & Daley, G. Q. (2013). Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell, 12, 395–406.
Kunitomi, A., Yuasa, S., Sugiyama, F., Saito, Y., Seki, T., Kusumoto, D., Kashimura, S., Takei, M., Tohyama, S., Hashimoto, H., Egashira, T., Tanimoto, Y., Mizuno, S., Tanaka, S., Okuno, H., Yamazawa, K., Watanabe, H., Oda, M., Kaneda, R., Matsuzaki, Y., Nagai, T., Okano, H., Yagami, K. I., Tanaka, M., & Fukuda, K. (2016). H1foo Has a Pivotal Role in Qualifying Induced Pluripotent Stem Cells. Stem Cell Reports, 6, 825–833.
Utikal, J., Polo, J. M., Stadtfeld, M., Maherali, N., Kulalert, W., Walsh, R. M., Khalil, A., Rheinwald, J. G., & Hochedlinger, K. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature, 460, 1145–1148.
Hong, H., Takahashi, K., Ichisaka, T., Aoi, T., Kanagawa, O., Nakagawa, M., Okita, K., & Yamanaka, S. (2009). Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature, 460, 1132–1135.
Ichida, J. K., Blanchard, J., Lam, K., Son, E. Y., Chung, J. E., Egli, D., Loh, K. M., Carter, A. C., Di Giorgio, F. P., Koszka, K., Huangfu, D., Akutsu, H., Liu, D. R., Rubin, L. L., & Eggan, K. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5, 491–503.
Maherali, N., & Hochedlinger, K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Current Biology, 19, 1718–1723.
Ying, Q. L., Wray, J., Nichols, J., Batlle-Morera, L., Doble, B., Woodgett, J., Cohen, P., & Smith, A. (2008). The ground state of embryonic stem cell self-renewal. Nature, 453, 519–523.
Chen, G., Guo, Y., Li, C., Li, S., & Wan, X. (2020). Small molecules that promote self-renewal of stem cells and somatic cell reprogramming. Stem Cell Reviews and Reports, 16, 511–523.
Mandai, M., Watanabe, A., Kurimoto, Y., Hirami, Y., Morinaga, C., Daimon, T., Fujihara, M., Akimaru, H., Sakai, N., Shibata, Y., Terada, M., Nomiya, Y., Tanishima, S., Nakamura, M., Kamao, H., Sugita, S., Onishi, A., Ito, T., Fujita, K., Kawamata, S., Go, M. J., Shinohara, C., Hata, K. I., Sawada, M., Yamamoto, M., Ohta, S., Ohara, Y., Yoshida, K., Kuwahara, J., Kitano, Y., Amano, N., Umekage, M., Kitaoka, F., Tanaka, A., Okada, C., Takasu, N., Ogawa, S., Yamanaka, S., & Takahashi, M. (2017). Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. The New England Journal of Medicine, 376, 1038–1046.
Takahashi, J. (2019). Preparing for first human trial of induced pluripotent stem cell-derived cells for Parkinson's disease: an interview with Jun Takahashi. Regenerative Medicine, 14, 93–95.
Huang, C. Y., Liu, C. L., Ting, C. Y., Chiu, Y. T., Cheng, Y. C., Nicholson, M. W., & Hsieh, P. C. H. (2019). Human iPSC banking: barriers and opportunities. Journal of Biomedical Science, 26, 87.
Hanna, J., Wernig, M., Markoulaki, S., Sun, C. W., Meissner, A., Cassady, J. P., Beard, C., Brambrink, T., Wu, L. C., Townes, T. M., & Jaenisch, R. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science, 318, 1920–1923.
Akita, H., Yoshie, S., Ishida, T., Takeishi, Y., & Hazama, A. (2020). Negative chronotropic and inotropic effects of lubiprostone on iPS cell-derived cardiomyocytes via activation of CFTR. BMC Complement Med Ther, 20, 118.
Jeon, K., Lim, H., Kim, J. H., Thuan, N. V., Park, S. H., Lim, Y. M., Choi, H. Y., Lee, E. R., Lee, M. S., & Cho, S. G. (2012). Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells and Development, 21, 2642–2655.
Maehr, R., Chen, S., Snitow, M., Ludwig, T., Yagasaki, L., Goland, R., Leibel, R. L., & Melton, D. A. (2009). Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America, 106, 15768–15773.
Pennarossa, G., Maffei, S., Campagnol, M., Tarantini, L., Gandolfi, F., & Brevini, T. A. (2013). Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proceedings of the National Academy of Sciences of the United States of America, 110, 8948–8953.
Tateishi, K., He, J., Taranova, O., Liang, G., D'Alessio, A. C., & Zhang, Y. (2008). Generation of insulin-secreting islet-like clusters from human skin fibroblasts. The Journal of Biological Chemistry, 283, 31601–31607.
Wang, R. M., & Christman, K. L. (2016). Decellularized Myocardial Matrix Hydrogels: In Basic Research and Preclinical Studies. Advanced Drug Delivery Reviews, 96, 77–82.
O'Neal, W. T., Griffin, W. F., Dries-Devlin, J. L., Kent, S. D., Chen, J., Willis, M. S., & Virag, J. A. (2013). Ephrin-Eph signaling as a potential therapeutic target for the treatment of myocardial infarction. Medical Hypotheses, 80, 738–744.
Ozawa, T., Mickle, D. A., Weisel, R. D., Koyama, N., Ozawa, S., & Li, R. K. (2002). Optimal biomaterial for creation of autologous cardiac grafts. Circulation, 106, 176–182.
Guan, X., Xu, W., Zhang, H., Wang, Q., Yu, J., Zhang, R., Chen, Y., Xia, Y., Wang, J., & Wang, D. (2020). Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Research & Therapy, 11, 73.
Ishida, M., Miyagawa, S., Saito, A., Fukushima, S., Harada, A., Ito, E., Ohashi, F., Watabe, T., Hatazawa, J., Matsuura, K., & Sawa, Y. (2019). Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation, 103, 291–298.
Caron, J., Pène, V., Tolosa, L., Villaret, M., Luce, E., Fourrier, A., Heslan, J. M., Saheb, S., Bruckert, E., Gómez-Lechón, M. J., Nguyen, T. H., Rosenberg, A. R., Weber, A., & Dubart-Kupperschmitt, A. (2019). Low-density lipoprotein receptor-deficient hepatocytes differentiated from induced pluripotent stem cells allow familial hypercholesterolemia modeling, CRISPR/Cas-mediated genetic correction, and productive hepatitis C virus infection. Stem Cell Research & Therapy, 10, 221.
Adamiak, M., Cheng, G., Bobis-Wozowicz, S., Zhao, L., Kedracka-Krok, S., Samanta, A., Karnas, E., Xuan, Y. T., Skupien-Rabian, B., Chen, X., Jankowska, U., Girgis, M., Sekula, M., Davani, A., Lasota, S., Vincent, R. J., Sarna, M., Newell, K. L., Wang, O. L., Dudley, N., Madeja, Z., Dawn, B., & Zuba-Surma, E. K. (2018). Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circulation Research, 122, 296–309.
Kodo, K., Ong, S. G., Jahanbani, F., Termglinchan, V., Hirono, K., InanlooRahatloo, K., Ebert, A. D., Shukla, P., Abilez, O. J., Churko, J. M., Karakikes, I., Jung, G., Ichida, F., Wu, S. M., Snyder, M. P., Bernstein, D., & Wu, J. C. (2016). iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nature Cell Biology, 18, 1031–1042.
Choi, I. Y., Lim, H., Estrellas, K., Mula, J., Cohen, T. V., Zhang, Y., Donnelly, C. J., Richard, J. P., Kim, Y. J., Kim, H., Kazuki, Y., Oshimura, M., Li, H. L., Hotta, A., Rothstein, J., Maragakis, N., Wagner, K. R., & Lee, G. (2016). Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myoblasts Derived using a Human iPSC-Based Model. Cell Reports, 15, 2301–2312.
Atchison, L., Zhang, H., Cao, K., & Truskey, G. A. (2017). A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells. Scientific Reports, 7, 8168.
Protze, S. I., Liu, J., Nussinovitch, U., Ohana, L., Backx, P. H., Gepstein, L., & Keller, G. M. (2017). Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nature Biotechnology, 35, 56–68.
Shiba, Y., Gomibuchi, T., Seto, T., Wada, Y., Ichimura, H., Tanaka, Y., Ogasawara, T., Okada, K., Shiba, N., Sakamoto, K., Ido, D., Shiina, T., Ohkura, M., Nakai, J., Uno, N., Kazuki, Y., Oshimura, M., Minami, I., & Ikeda, U. (2016). Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature, 538, 388–391.
Funakoshi, S., Miki, K., Takaki, T., Okubo, C., Hatani, T., Chonabayashi, K., Nishikawa, M., Takei, I., Oishi, A., Narita, M., Hoshijima, M., Kimura, T., Yamanaka, S., & Yoshida, Y. (2016). Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Scientific Reports, 6, 19111.
Zhou, Y., Wang, S., Yu, Z., Hoyt, R. F. J., Horvath, K. A., & Singh, A. K. (2012). Allogeneic Transplantation of Induced Pluripotent Stem Cells in a Porcine Model of Chronic Myocardial Ischemia Failed to Stimulate Myocyte Differentiation: 1649. Transplantation, 94, 1014.
Sun, N., Yazawa, M., Liu, J., Han, L., Sanchez-Freire, V., Abilez, O. J., Navarrete, E. G., Hu, S., Wang, L., Lee, A., Pavlovic, A., Lin, S., Chen, R., Hajjar, R. J., Snyder, M. P., Dolmetsch, R. E., Butte, M. J., Ashley, E. A., Longaker, M. T., Robbins, R. C., & Wu, J. C. (2012). Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science Translational Medicine, 4, 130ra147.
Itzhaki, I., Maizels, L., Huber, I., Zwi-Dantsis, L., Caspi, O., Winterstern, A., Feldman, O., Gepstein, A., Arbel, G., Hammerman, H., Boulos, M., & Gepstein, L. (2011). Modelling the long QT syndrome with induced pluripotent stem cells. Nature, 471, 225–229.
Sala, L., Gnecchi, M., & Schwartz, P. J. (2019). Long QT Syndrome Modelling with Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells. Arrhythmia & Electrophysiology Review, 8, 105–110.
Paik, D. T., Chandy, M., & Wu, J. C. (2020). Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacological Reviews, 72, 320–342.
Parrotta, E. I., Lucchino, V., Scaramuzzino, L., Scalise, S., & Cuda, G. (2020). Modeling Cardiac Disease Mechanisms Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Progress, Promises and Challenges. International Journal of Molecular Sciences, 21.
Carpenter, L., Carr, C., Yang, C. T., Stuckey, D. J., Clarke, K., & Watt, S. M. (2012). Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells and Development, 21, 977–986.
Gu, M., Nguyen, P. K., Lee, A. S., Xu, D., Hu, S., Plews, J. R., Han, L., Huber, B. C., Lee, W. H., Gong, Y., de Almeida, P. E., Lyons, J., Ikeno, F., Pacharinsak, C., Connolly, A. J., Gambhir, S. S., Robbins, R. C., Longaker, M. T., & Wu, J. C. (2012). Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circulation Research, 111, 882–893.
Lee, A. S., Xu, D., Plews, J. R., Nguyen, P. K., Nag, D., Lyons, J. K., Han, L., Hu, S., Lan, F., Liu, J., Huang, M., Narsinh, K. H., Long, C. T., de Almeida, P. E., Levi, B., Kooreman, N., Bangs, C., Pacharinsak, C., Ikeno, F., Yeung, A. C., Gambhir, S. S., Robbins, R. C., Longaker, M. T., & Wu, J. C. (2011). Preclinical derivation and imaging of autologously transplanted canine induced pluripotent stem cells. The Journal of Biological Chemistry, 286, 32697–32704.
Tachibana, A., Santoso, M. R., Mahmoudi, M., Shukla, P., Wang, L., Bennett, M., Goldstone, A. B., Wang, M., Fukushi, M., Ebert, A. D., Woo, Y. J., Rulifson, E., & Yang, P. C. (2017). Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circulation Research, 121, e22–e36.
Templin, C., Zweigerdt, R., Schwanke, K., Olmer, R., Ghadri, J. R., Emmert, M. Y., Müller, E., Küest, S. M., Cohrs, S., Schibli, R., Kronen, P., Hilbe, M., Reinisch, A., Strunk, D., Haverich, A., Hoerstrup, S., Lüscher, T. F., Kaufmann, P. A., Landmesser, U., & Martin, U. (2012). Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation, 126, 430–439.
Ye, L., Chang, Y. H., Xiong, Q., Zhang, P., Zhang, L., Somasundaram, P., Lepley, M., Swingen, C., Su, L., Wendel, J. S., Guo, J., Jang, A., Rosenbush, D., Greder, L., Dutton, J. R., Zhang, J., Kamp, T. J., Kaufman, D. S., & Ge, Y. (2014). Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell, 15, 750–761.
Li, L., Song, Y., Shi, X., Liu, J., Xiong, S., Chen, W., Fu, Q., Huang, Z., Gu, N., & Zhang, R. (2018). The landscape of miRNA editing in animals and its impact on miRNA biogenesis and targeting. Genome Research, 28, 132–143.
Cyranoski, D. (2018). [online] Available at: https://www.nature.com/articles/d41586-018-05278-8. Nature, 2018. Accessed 03 Oct. 2020.
Kadota, S., & Shiba, Y. (2019). Pluripotent Stem Cell-Derived Cardiomyocyte Transplantation for Heart Disease Treatment. Current Cardiology Reports, 21, 73.
Engleka, K. A., Manderfield, L. J., Brust, R. D., Li, L., Cohen, A., Dymecki, S. M., & Epstein, J. A. (2012). Islet1 derivatives in the heart are of both neural crest and second heart field origin. Circulation Research, 110, 922–926.
Ge, Z., Lal, S., Le, T. Y. L., Dos Remedios, C., & Chong, J. J. H. (2015). Cardiac stem cells: translation to human studies. Biophysical Reviews, 7, 127–139.
Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., Nakaya, H., Kasanuki, H., & Komuro, I. (2004). Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. The Journal of Biological Chemistry, 279, 11384–11391.
Serradifalco, C., Catanese, P., Rizzuto, L., Cappello, F., Puleio, R., Barresi, V., Nunnari, C. M., Zummo, G., & Di Felice, V. (2011). Embryonic and foetal Islet-1 positive cells in human hearts are also positive to c-Kit. European Journal of Histochemistry, 55, 229–234.
Avolio, E., Meloni, M., Spencer, H. L., Riu, F., Katare, R., Mangialardi, G., Oikawa, A., Rodriguez-Arabaolaza, I., Dang, Z., Mitchell, K., Reni, C., Alvino, V. V., Rowlinson, J., Livi, U., Cesselli, D., Angelini, G., Emanueli, C., Beltrami, A. P., & Madeddu, P. (2015). Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circulation Research, 116, e81–e94.
Santini, M. P., Forte, E., Harvey, R. P., & Kovacic, J. C. (2016). Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development, 143, 1242–1258.
Domian, I. J., Chiravuri, M., van der Meer, P., Feinberg, A. W., Shi, X., Shao, Y., Wu, S. M., Parker, K. K., & Chien, K. R. (2009). Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science, 326, 426–429.
Gaetani, R., Doevendans, P. A., Metz, C. H., Alblas, J., Messina, E., Giacomello, A., & Sluijter, J. P. (2012). Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials, 33, 1782–1790.
Senyo, S. E., Steinhauser, M. L., Pizzimenti, C. L., Yang, V. K., Cai, L., Wang, M., Wu, T. D., Guerquin-Kern, J. L., Lechene, C. P., & Lee, R. T. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature, 493, 433–436.
Parrag, I. C., Zandstra, P. W., & Woodhouse, K. A. (2012). Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnology and Bioengineering, 109, 813–822.
Mandel, Y., Weissman, A., Schick, R., Barad, L., Novak, A., Meiry, G., Goldberg, S., Lorber, A., Rosen, M. R., Itskovitz-Eldor, J., & Binah, O. (2012). Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation, 125, 883–893.
Parsons, X. H., Teng, Y. D., Moore, D. A., & Snyder, E. Y. (2011). Patents on Technologies of Human Tissue and Organ Regeneration from Pluripotent Human Embryonic Stem Cells. Rec Pat Regen Med, 1, 142–163.
Shen, N., Knopf, A., Westendorf, C., Kraushaar, U., Riedl, J., Bauer, H., Poschel, S., Layland, S. L., Holeiter, M., Knolle, S., Brauchle, E., Nsair, A., Hinderer, S., & Schenke-Layland, K. (2017). Steps toward Maturation of Embryonic Stem Cell-Derived Cardiomyocytes by Defined Physical Signals. Stem Cell Reports, 9, 122–135.
Liau, B., Christoforou, N., Leong, K. W., & Bursac, N. (2011). Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials, 32, 9180–9187.
Moon, S. H., Kang, S. W., Park, S. J., Bae, D., Kim, S. J., Lee, H. A., Kim, K. S., Hong, K. S., Kim, J. S., Do, J. T., Byun, K. H., & Chung, H. M. (2013). The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials, 34, 4013–4026.
Guo, X. M., Zhao, Y. S., Chang, H. X., Wang, C. Y., E, L.-L., Zhang, X. A., Duan, C. M., Dong, L. Z., Jiang, H., Li, J., Song, Y., & Yang, X. J. (2006). Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation, 113, 2229–2237.
Hynes, R. O. (2008). US policies on human embryonic stem cells. Nature Reviews. Molecular Cell Biology, 9, 993–997.
Gupta, M. K., Uhm, S. J., Lee, S. H., & Lee, H. T. (2008). Role of nonessential amino acids on porcine embryos produced by parthenogenesis or somatic cell nuclear transfer. Molecular Reproduction and Development, 75, 588–597.
Ju, J. Y., Park, C. Y., Gupta, M. K., Uhm, S. J., Paik, E. C., Ryoo, Z. Y., Cho, Y. H., Chung, K. S., & Lee, H. T. (2008). Establishment of stem cell lines from nuclear transferred and parthenogenetically activated mouse oocytes for therapeutic cloning. Fertility and Sterility, 89, 1314–1323.
Hare, J. M., Traverse, J. H., Henry, T. D., Dib, N., Strumpf, R. K., Schulman, S. P., Gerstenblith, G., DeMaria, A. N., Denktas, A. E., Gammon, R. S., Hermiller Jr., J. B., Reisman, M. A., Schaer, G. L., & Sherman, W. (2009). A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology, 54, 2277–2286.
Abd Emami, B., Mahmoudi, E., Shokrgozar, M. A., Dehghan, M. M., Farzad Mohajeri, S., Haghighipour, N., Marjanmehr, S. H., Molazem, M., Amin, S., & Gholami, H. (2018). Mechanical and Chemical Predifferentiation of Mesenchymal Stem Cells Into Cardiomyocytes and Their Effectiveness on Acute Myocardial Infarction. Artificial Organs, 42, E114–E126.
Cai, M., Shen, R., Song, L., Lu, M., Wang, J., Zhao, S., Tang, Y., Meng, X., Li, Z., & He, Z. X. (2016). Bone Marrow Mesenchymal Stem Cells (BM-MSCs) Improve Heart Function in Swine Myocardial Infarction Model through Paracrine Effects. Scientific Reports, 6, 28250.
Gerace, D., Martiniello-Wilks, R., Habib, R., Ren, B., Nassif, N. T., O'Brien, B. A., & Simpson, A. M. (2019). Ex Vivo Expansion of Murine MSC Impairs Transcription Factor-Induced Differentiation into Pancreatic beta-Cells. Stem Cells International, 2019, 1395301.
Li, G., Chen, J., Zhang, X., He, G., Tan, W., Wu, H., Li, R., Chen, Y., Gu, R., Xie, J., & Xu, B. (2017). Cardiac repair in a mouse model of acute myocardial infarction with trophoblast stem cells. Scientific Reports, 7, 44376.
Planat-Benard, V., Menard, C., Andre, M., Puceat, M., Perez, A., Garcia-Verdugo, J. M., Penicaud, L., & Casteilla, L. (2004). Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circulation Research, 94, 223–229.
Van Dijk, A., Niessen, H. W., Zandieh Doulabi, B., Visser, F. C., & van Milligen, F. J. (2008). Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell and Tissue Research, 334, 457–467.
Smith, A. W., Segar, C. E., Nguyen, P. K., MacEwan, M. R., Efimov, I. R., & Elbert, D. L. (2012). Long-term culture of HL-1 cardiomyocytes in modular poly(ethylene glycol) microsphere-based scaffolds crosslinked in the phase-separated state. Acta Biomaterialia, 8, 31–40.
Shiota, M., Heike, T., Haruyama, M., Baba, S., Tsuchiya, A., Fujino, H., Kobayashi, H., Kato, T., Umeda, K., Yoshimoto, M., & Nakahata, T. (2007). Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Experimental Cell Research, 313, 1008–1023.
Homayouni Moghadam, F., Tayebi, T., & Barzegar, K. (2016). Differentiation of Rat bone marrow Mesenchymal stem cells into Adipocytes and Cardiomyocytes after treatment with platelet lysate. Int J Hematol Oncol Stem Cell Res, 10, 21–29.
Rouhi, L., Kajbafzadeh, A. M., Modaresi, M., Shariati, M., & Hamrahi, D. (2013). Autologous serum enhances cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells in the presence of transforming growth factor-beta1 (TGF-beta1). In Vitro Cellular & Developmental Biology. Animal, 49, 287–294.
Xaymardan, M., Tang, L., Zagreda, L., Pallante, B., Zheng, J., Chazen, J. L., Chin, A., Duignan, I., Nahirney, P., Rafii, S., Mikawa, T., & Edelberg, J. M. (2004). Platelet-derived growth factor-AB promotes the generation of adult bone marrow-derived cardiac myocytes. Circulation Research, 94, E39–E45.
Jumabay, M., Matsumoto, T., Yokoyama, S., Kano, K., Kusumi, Y., Masuko, T., Mitsumata, M., Saito, S., Hirayama, A., Mugishima, H., & Fukuda, N. (2009). Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. Journal of Molecular and Cellular Cardiology, 47, 565–575.
Choi, Y. S., Dusting, G. J., Stubbs, S., Arunothayaraj, S., Han, X. L., Collas, P., Morrison, W. A., & Dilley, R. J. (2010). Differentiation of human adipose-derived stem cells into beating cardiomyocytes. Journal of Cellular and Molecular Medicine, 14, 878–889.
Choi, Y. S., Matsuda, K., Dusting, G. J., Morrison, W. A., & Dilley, R. J. (2010). Engineering cardiac tissue in vivo from human adipose-derived stem cells. Biomaterials, 31, 2236–2242.
Mirotsou, M., Zhang, Z., Deb, A., Zhang, L., Gnecchi, M., Noiseux, N., Mu, H., Pachori, A., & Dzau, V. (2007). Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences of the United States of America, 104, 1643–1648.
Jawad, H., Ali, N. N., Lyon, A. R., Chen, Q. Z., Harding, S. E., & Boccaccini, A. R. (2007). Myocardial tissue engineering: A review. Journal of Tissue Engineering and Regenerative Medicine, 1, 327–342.
Pushp, P., Sahoo, B., Ferreira, F. C., Sampaio Cabral, J. M., Fernandes-Platzgummer, A., & Gupta, M. K. (2019). Functional comparison of beating cardiomyocytes differentiated from umbilical cord-derived mesenchymal/stromal stem cells and human foreskin-derived induced pluripotent stem cells. Journal of Biomedical Materials Research Part A, 108, 496–514.
Baba, S., Heike, T., Umeda, K., Iwasa, T., Kaichi, S., Hiraumi, Y., Doi, H., Yoshimoto, M., Kanatsu-Shinohara, M., Shinohara, T., & Nakahata, T. (2007). Generation of cardiac and endothelial cells from neonatal mouse testis-derived multipotent germline stem cells. Stem Cells, 25, 1375–1383.
Guan, K., Nayernia, K., Maier, L. S., Wagner, S., Dressel, R., Lee, J. H., Nolte, J., Wolf, F., Li, M., Engel, W., & Hasenfuss, G. (2006). Pluripotency of spermatogonial stem cells from adult mouse testis. Nature, 440, 1199–1203.
Kanatsu-Shinohara, M., Miki, H., Inoue, K., Ogonuki, N., Toyokuni, S., Ogura, A., & Shinohara, T. (2005). Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biology of Reproduction, 72, 985–991.
Ko, K., Tapia, N., Wu, G., Kim, J. B., Bravo, M. J., Sasse, P., Glaser, T., Ruau, D., Han, D. W., Greber, B., Hausdorfer, K., Sebastiano, V., Stehling, M., Fleischmann, B. K., Brustle, O., Zenke, M., & Scholer, H. R. (2009). Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell, 5, 87–96.
Iwasa, T., Baba, S., Doi, H., Kaichi, S., Yokoo, N., Mima, T., Kanatsu-Shinohara, M., Shinohara, T., Nakahata, T., & Heike, T. (2010). Neonatal mouse testis-derived multipotent germline stem cells improve the cardiac function of acute ischemic heart mouse model. Biochemical and Biophysical Research Communications, 400, 27–33.
Guan, K., Wagner, S., Unsold, B., Maier, L. S., Kaiser, D., Hemmerlein, B., Nayernia, K., Engel, W., & Hasenfuss, G. (2007). Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circulation Research, 100, 1615–1625.
Nguyen, T. L., Yoo, J. G., Sharma, N., Kim, S. W., Kang, Y. J., Thi, H. H. P., & Jeong, D. K. (2016). Isolation, characterization and differentiation potential of chicken spermatogonial stem cell derived embryoid bodies. Annals of Animal Science, 16, 115–128.
Jung, Y. H., Gupta, M. K., Oh, S. H., Uhm, S. J., & Lee, H. T. (2010). Glial cell line-derived neurotrophic factor alters the growth characteristics and genomic imprinting of mouse multipotent adult germline stem cells. Experimental Cell Research, 316, 747–761.
Lim, J. M., & Gong, S. P. (2013). Somatic cell transformation into stem cell-like cells induced by different microenvironments. Organogenesis, 9, 245–248.
Graichen, R., Xu, X., Braam, S. R., Balakrishnan, T., Norfiza, S., Sieh, S., Soo, S. Y., Tham, S. C., Mummery, C., Colman, A., Zweigerdt, R., & Davidson, B. P. (2008). Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation, 76, 357–370.
Matsuda, Y., Takahashi, K., Kamioka, H., & Naruse, K. (2018). Human gingival fibroblast feeder cells promote maturation of induced pluripotent stem cells into cardiomyocytes. Biochemical and Biophysical Research Communications, 503, 1798–1804.
Fujiwara, M., Yan, P., Otsuji, T. G., Narazaki, G., Uosaki, H., Fukushima, H., Kuwahara, K., Harada, M., Matsuda, H., Matsuoka, S., Okita, K., Takahashi, K., Nakagawa, M., Ikeda, T., Sakata, R., Mummery, C. L., Nakatsuji, N., Yamanaka, S., Nakao, K., & Yamashita, J. K. (2011). Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One, 6, e16734.
Laco, F., Lam, A. T., Woo, T. L., Tong, G., Ho, V., Soong, P. L., Grishina, E., Lin, K. H., Reuveny, S., & Oh, S. K. (2020). Selection of human induced pluripotent stem cells lines optimization of cardiomyocytes differentiation in an integrated suspension microcarrier bioreactor. Stem Cell Research & Therapy, 11, 118.
Chen, V. C., Ye, J., Shukla, P., Hua, G., Chen, D., Lin, Z., Liu, J. C., Chai, J., Gold, J., Wu, J., Hsu, D., & Couture, L. A. (2015). Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Research, 15, 365–375.
Fonoudi, H., Ansari, H., Abbasalizadeh, S., Larijani, M. R., Kiani, S., Hashemizadeh, S., Zarchi, A. S., Bosman, A., Blue, G. M., Pahlavan, S., Perry, M., Orr, Y., Mayorchak, Y., Vandenberg, J., Talkhabi, M., Winlaw, D. S., Harvey, R. P., Aghdami, N., & Baharvand, H. (2015). A Universal and Robust Integrated Platform for the Scalable Production of Human Cardiomyocytes From Pluripotent Stem Cells. Stem Cells Translational Medicine, 4, 1482–1494.
Halloin, C., Schwanke, K., Lobel, W., Franke, A., Szepes, M., Biswanath, S., Wunderlich, S., Merkert, S., Weber, N., Osten, F., de la Roche, J., Polten, F., Christoph Wollert, K., Kraft, T., Fischer, M., Martin, U., Gruh, I., Kempf, H., & Zweigerdt, R. (2019). Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Reports, 13, 366–379.
Titmarsh, D. M., Glass, N. R., Mills, R. J., Hidalgo, A., Wolvetang, E. J., Porrello, E. R., Hudson, J. E., & Cooper-White, J. J. (2016). Induction of Human iPSC-Derived Cardiomyocyte Proliferation Revealed by Combinatorial Screening in High Density Microbioreactor Arrays. Scientific Reports, 6, 24637.
Wang, G., McCain, M. L., Yang, L., He, A., Pasqualini, F. S., Agarwal, A., Yuan, H., Jiang, D., Zhang, D., Zangi, L., Geva, J., Roberts, A. E., Ma, Q., Ding, J., Chen, J., Wang, D. Z., Li, K., Wang, J., Wanders, R. J., Kulik, W., Vaz, F. M., Laflamme, M. A., Murry, C. E., Chien, K. R., Kelley, R. I., Church, G. M., Parker, K. K., & Pu, W. T. (2014). Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature Medicine, 20, 616–623.
Kattman, S. J., Witty, A. D., Gagliardi, M., Dubois, N. C., Niapour, M., Hotta, A., Ellis, J., & Keller, G. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell, 8, 228–240.
Chen, Y., Zeng, D., Ding, L., Li, X. L., Liu, X. T., Li, W. J., Wei, T., Yan, S., Xie, J. H., Wei, L., & Zheng, Q. S. (2015). Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biology, 16, 22.
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., Plews, J. R., Abilez, O. J., Cui, B., Gold, J. D., & Wu, J. C. (2014). Chemically defined generation of human cardiomyocytes. Nature Methods, 11, 855–860.
Hatani, T., Miki, K., & Yoshida, Y. (2018). Induction of Human Induced Pluripotent Stem Cells to Cardiomyocytes Using Embryoid Bodies. Methods in Molecular Biology, 1816, 79–92.
Miki, K., Uenaka, H., Saito, A., Miyagawa, S., Sakaguchi, T., Higuchi, T., Shimizu, T., Okano, T., Yamanaka, S., & Sawa, Y. (2012). Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Translational Medicine, 1, 430–437.
Lewandowski, J., Kolanowski, T. J., & Kurpisz, M. (2017). Techniques for the induction of human pluripotent stem cell differentiation towards cardiomyocytes. Journal of Tissue Engineering and Regenerative Medicine, 11, 1658–1674.
Uosaki, H., Fukushima, H., Takeuchi, A., Matsuoka, S., Nakatsuji, N., Yamanaka, S., & Yamashita, J. K. (2011). Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One, 6, e23657.
Dubois, N. C., Craft, A. M., Sharma, P., Elliott, D. A., Stanley, E. G., Elefanty, A. G., Gramolini, A., & Keller, G. (2011). SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature Biotechnology, 29, 1011–1018.
Miki, K., Endo, K., Takahashi, S., Funakoshi, S., Takei, I., Katayama, S., Toyoda, T., Kotaka, M., Takaki, T., Umeda, M., Okubo, C., Nishikawa, M., Oishi, A., Narita, M., Miyashita, I., Asano, K., Hayashi, K., Osafune, K., Yamanaka, S., Saito, H., & Yoshida, Y. (2015). Efficient Detection and Purification of Cell Populations Using Synthetic MicroRNA Switches. Cell Stem Cell, 16, 699–711.
Tohyama, S., Hattori, F., Sano, M., Hishiki, T., Nagahata, Y., Matsuura, T., Hashimoto, H., Suzuki, T., Yamashita, H., Satoh, Y., Egashira, T., Seki, T., Muraoka, N., Yamakawa, H., Ohgino, Y., Tanaka, T., Yoichi, M., Yuasa, S., Murata, M., Suematsu, M., & Fukuda, K. (2013). Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell, 12, 127–137.
Tohyama, S., Fujita, J., Hishiki, T., Matsuura, T., Hattori, F., Ohno, R., Kanazawa, H., Seki, T., Nakajima, K., Kishino, Y., Okada, M., Hirano, A., Kuroda, T., Yasuda, S., Sato, Y., Yuasa, S., Sano, M., Suematsu, M., & Fukuda, K. (2016). Glutamine Oxidation Is Indispensable for Survival of Human Pluripotent Stem Cells. Cell Metabolism, 23, 663–674.
Zhang, J. Z., Termglinchan, V., Shao, N. Y., Itzhaki, I., Liu, C., Ma, N., Tian, L., Wang, V. Y., Chang, A. C. Y., Guo, H., Kitani, T., Wu, H., Lam, C. K., Kodo, K., Sayed, N., Blau, H. M., & Wu, J. C. (2019). A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles. Cell Stem Cell, 24, 802–811.e805.
Devalla, H. D., Schwach, V., Ford, J. W., Milnes, J. T., El-Haou, S., Jackson, C., Gkatzis, K., Elliott, D. A., & Chuva de Sousa Lopes, S.M., Mummery, C.L., Verkerk, A.O. & Passier, R. (2015). Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Molecular Medicine, 7, 394–410.
Guo, Y., & Pu, W. T. (2020). Cardiomyocyte Maturation: New Phase in Development. Circulation Research, 126, 1086–1106.
Yang, X., Pabon, L., & Murry, C. E. (2014). Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation Research, 114, 511–523.
Ahmed, R. E., Anzai, T., Chanthra, N., & Uosaki, H. (2020). A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Frontiers in Cell and Development Biology, 8, 178.
Lundy, S. D., Zhu, W. Z., Regnier, M., & Laflamme, M. A. (2013). Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells and Development, 22, 1991–2002.
Zhu, R., Blazeski, A., Poon, E., Costa, K. D., Tung, L., & Boheler, K. R. (2014). Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Research & Therapy, 5, 117.
Correia, C., Koshkin, A., Duarte, P., Hu, D., Teixeira, A., Domian, I., Serra, M., & Alves, P. M. (2017). Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Scientific Reports, 7, 8590.
Kikuchi, C., Bienengraeber, M., Canfield, S., Koopmeiner, A., Schäfer, R., Bosnjak, Z. J., & Bai, X. (2015). Comparison of Cardiomyocyte Differentiation Potential Between Type 1 Diabetic Donor- and Nondiabetic Donor-Derived Induced Pluripotent Stem Cells. Cell Transplantation, 24, 2491–2504.
Dias, T. P., Pinto, S. N., Santos, J. I., Fernandes, T. G., Fernandes, F., Diogo, M. M., Prieto, M., & Cabral, J. M. S. (2018). Biophysical study of human induced Pluripotent Stem Cell-Derived cardiomyocyte structural maturation during long-term culture. Biochemical and Biophysical Research Communications, 499, 611–617.
Gentillon, C., Li, D., Duan, M., Yu, W. M., Preininger, M. K., Jha, R., Rampoldi, A., Saraf, A., Gibson, G. C., Qu, C. K., Brown, L. A., & Xu, C. (2019). Targeting HIF-1α in combination with PPARα activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. Journal of Molecular and Cellular Cardiology, 132, 120–135.
Parikh, S. S., Blackwell, D. J., Gomez-Hurtado, N., Frisk, M., Wang, L., Kim, K., Dahl, C. P., Fiane, A., Tønnessen, T., Kryshtal, D. O., Louch, W. E., & Knollmann, B. C. (2017). Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation Research, 121, 1323–1330.
Dunn, K. K., Reichardt, I. M., Simmons, A. D., Jin, G., Floy, M. E., Hoon, K. M., & Palecek, S. P. (2019). Coculture of Endothelial Cells with Human Pluripotent Stem Cell-Derived Cardiac Progenitors Reveals a Differentiation Stage-Specific Enhancement of Cardiomyocyte Maturation. Biotechnology Journal, 14, e1800725.
Yoshida, S., Miyagawa, S., Fukushima, S., Kawamura, T., Kashiyama, N., Ohashi, F., Toyofuku, T., Toda, K., & Sawa, Y. (2018). Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Molecular Therapy, 26, 2681–2695.
Herron, T. J., Rocha, A. M., Campbell, K. F., Ponce-Balbuena, D., Willis, B. C., Guerrero-Serna, G., Liu, Q., Klos, M., Musa, H., Zarzoso, M., Bizy, A., Furness, J., Anumonwo, J., Mironov, S., & Jalife, J. (2016). Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circulation. Arrhythmia and Electrophysiology, 9, e003638.
Huang, C. Y., Peres Moreno Maia-Joca, R., Ong, C. S., Wilson, I., DiSilvestre, D., Tomaselli, G. F., & Reich, D. H. (2020). Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. Journal of Molecular and Cellular Cardiology, 138, 1–11.
Branco, M. A., Cotovio, J. P., Rodrigues, C. A. V., Vaz, S. H., Fernandes, T. G., Moreira, L. M., Cabral, J. M. S., & Diogo, M. M. (2019). Transcriptomic analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells Reveals Faster Cardiomyocyte Maturation Compared to 2D Culture. Scientific Reports, 9, 9229.
Nunes, S. S., Miklas, J. W., Liu, J., Aschar-Sobbi, R., Xiao, Y., Zhang, B., Jiang, J., Massé, S., Gagliardi, M., Hsieh, A., Thavandiran, N., Laflamme, M. A., Nanthakumar, K., Gross, G. J., Backx, P. H., Keller, G., & Radisic, M. (2013). Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature Methods, 10, 781–787.
Ebert, A., Joshi, A. U., Andorf, S., Dai, Y., Sampathkumar, S., Chen, H., Li, Y., Garg, P., Toischer, K., Hasenfuss, G., Mochly-Rosen, D., & Wu, J. C. (2019). Proteasome-Dependent Regulation of Distinct Metabolic States During Long-Term Culture of Human iPSC-Derived Cardiomyocytes. Circulation Research, 125, 90–103.
Kolanowski, T. J., Busek, M., Schubert, M., Dmitrieva, A., Binnewerg, B., Pöche, J., Fisher, K., Schmieder, F., Grünzner, S., Hansen, S., Richter, A., El-Armouche, A., Sonntag, F., & Guan, K. (2020). Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system. Acta Biomaterialia, 102, 273–286.
Pekkanen-Mattila, M., Häkli, M., Pölönen, R. P., Mansikkala, T., Junnila, A., Talvitie, E., Koivisto, J. T., Kellomäki, M., & Aalto-Setälä, K. (2019). Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials (Basel), 12, 1805.
Di Baldassarre, A., Cimetta, E., Bollini, S., Gaggi, G., & Ghinassi, B. (2018). Human-Induced Pluripotent Stem Cell Technology and Cardiomyocyte Generation: Progress and Clinical Applications. Cells, 7, 48.
Reinecke, H., Zhang, M., Bartosek, T., & Murry, C. E. (1999). Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation, 100, 193–202.
Karbassi, E., Fenix, A., Marchiano, S., Muraoka, N., Nakamura, K., Yang, X., & Murry, C. E. (2020). Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nature Reviews. Cardiology, 17, 341–359.
Li, J., Zhu, K., Yang, S., Wang, Y., Guo, C., Yin, K., Wang, C., & Lai, H. (2015). Fibrin patch-based insulin-like growth factor-1 gene-modified stem cell transplantation repairs ischemic myocardium. Experimental Biology and Medicine, 240, 585–592.
Ong, S. G., Huber, B. C., Lee, W. H., Kodo, K., Ebert, A. D., Ma, Y., Nguyen, P. K., Diecke, S., Chen, W. Y., & Wu, J. C. (2015). Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation, 132, 762–771.
Chow, A., Stuckey, D. J., Kidher, E., Rocco, M., Jabbour, R. J., Mansfield, C. A., Darzi, A., Harding, S. E., Stevens, M. M., & Athanasiou, T. (2017). Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Reports, 9, 1415–1422.
Wang, X., Chun, Y. W., Zhong, L., Chiusa, M., Balikov, D. A., Frist, A. Y., Lim, C. C., Maltais, S., Bellan, L., Hong, C. C., & Sung, H. J. (2015). A temperature-sensitive, self-adhesive hydrogel to deliver iPSC-derived cardiomyocytes for heart repair. International Journal of Cardiology, 190, 177–180.
Tabei, R., Kawaguchi, S., Kanazawa, H., Tohyama, S., Hirano, A., Handa, N., Hishikawa, S., Teratani, T., Kunita, S., Fukuda, J., Mugishima, Y., Suzuki, T., Nakajima, K., Seki, T., Kishino, Y., Okada, M., Yamazaki, M., Okamoto, K., Shimizu, H., Kobayashi, E., Tabata, Y., Fujita, J., & Fukuda, K. (2019). Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell–derived cardiomyocytes. The Journal of Heart and Lung Transplantation, 38, 203–214.
Ishigami, M., Masumoto, H., Ikuno, T., Aoki, T., Kawatou, M., Minakata, K., Ikeda, T., Sakata, R., Yamashita, J. K., & Minatoya, K. (2018). Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS One, 13, e0201650.
Kawamura, M., Miyagawa, S., Miki, K., Saito, A., Fukushima, S., Higuchi, T., Kawamura, T., Kuratani, T., Daimon, T., Shimizu, T., Okano, T., & Sawa, Y. (2012). Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation, 126, S29–S37.
Chang, D., Wen, Z., Wang, Y., Cai, W., Wani, M., Paul, C., Okano, T., & Millard, R. W. (2014). Ultrastructural features of ischemic tissue following application of a bio-membrane based progenitor cardiomyocyte patch for myocardial infarction repair. PLoS One, 9, e107296.
Komae, H., Sekine, H., Dobashi, I., Matsuura, K., Ono, M., Okano, T., & Shimizu, T. (2017). Three-dimensional functional human myocardial tissues fabricated from induced pluripotent stem cells. Journal of Tissue Engineering and Regenerative Medicine, 11, 926–935.
Seta, H., Matsuura, K., Sekine, H., Yamazaki, K., & Shimizu, T. (2017). Tubular Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells Generate Pulse Pressure In Vivo. Scientific Reports, 7, 45499.
Shadrin, I. Y., Allen, B. W., Qian, Y., Jackman, C. P., Carlson, A. L., Juhas, M. E., & Bursac, N. (2017). Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nature Communications, 8, 1825.
Wendel, J. S., Ye, L., Tao, R., Zhang, J., Kamp, T. J., & Tranquillo, R. T. (2015). Functional Effects of a Tissue-Engineered Cardiac Patch From Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Rat Infarct Model. Stem Cells Translational Medicine, 4, 1324–1332.
Weinberger, F., Breckwoldt, K., Pecha, S., Kelly, A., Geertz, B., Starbatty, J., Yorgan, T., Cheng, K. H., Lessmann, K., Stolen, T., Scherrer-Crosbie, M., Smith, G., Reichenspurner, H., Hansen, A., & Eschenhagen, T. (2016). Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Science Translational Medicine, 8, 363ra148.
Li, H., Bao, M., & Nie, Y. (2020). Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Failure Reviews. https://doi.org/10.1007/s10741-020-09953-9.
Xu, Y., Chen, C., Hellwarth, P. B., & Bao, X. (2019). Biomaterials for stem cell engineering and biomanufacturing. Bioact Mater, 4, 366–379.
Rao, C., Prodromakis, T., Kolker, L., Chaudhry, U. A., Trantidou, T., Sridhar, A., Weekes, C., Camelliti, P., Harding, S. E., Darzi, A., Yacoub, M. H., Athanasiou, T., & Terracciano, C. M. (2013). The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials, 34, 2399–2411.
Maiullari, F., Costantini, M., Milan, M., Pace, V., Chirivì, M., Maiullari, S., Rainer, A., Baci, D., Marei, H. E., Seliktar, D., Gargioli, C., Bearzi, C., & Rizzi, R. (2018). A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Scientific Reports, 8, 13532.
Schubert, M., Binnewerg, B., Voronkina, A., Muzychka, L., Wysokowski, M., Petrenko, I., Kovalchuk, V., Tsurkan, M., Martinovic, R., Bechmann, N., Ivanenko, V. N., Fursov, A., Smolii, O. B., Fromont, J., Joseph, Y., Bornstein, S. R., Giovine, M., Erpenbeck, D., Guan, K., & Ehrlich, H. (2019). Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). International Journal of Molecular Sciences, 20, 5105.
Song, X., Mei, J., Ye, G., Wang, L., Ananth, A., Yu, L., & Qiu, X. (2019). In situ pPy-modification of chitosan porous membrane from mussel shell as a cardiac patch to repair myocardial infarction. Applied Materials Today, 15, 87–99.
Liu, Y., Xu, Y., Wang, Z., Wen, D., Zhang, W., Schmull, S., Li, H., Chen, Y., & Xue, S. (2016). Electrospun nanofibrous sheets of collagen/elastin/polycaprolactone improve cardiac repair after myocardial infarction. American Journal of Translational Research, 8, 1678–1694.
Bejleri, D., Streeter, B. W., Nachlas, A. L. Y., Brown, M. E., Gaetani, R., Christman, K. L., & Davis, M. E. (2018). A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Advanced Healthcare Materials, 7, e1800672.
Shah, M., Kc, P., & Zhang, G. (2019). In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Applied Materials & Interfaces, 11, 23893–23900.
Fong, A. H., Romero-López, M., Heylman, C. M., Keating, M., Tran, D., Sobrino, A., Tran, A. Q., Pham, H. H., Fimbres, C., Gershon, P. D., Botvinick, E. L., George, S. C., & Hughes, C. C. (2016). Three-Dimensional Adult Cardiac Extracellular Matrix Promotes Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Tissue Engineering. Part A, 22, 1016–1025.
Kaiser, N. J., Kant, R. J., Minor, A. J., & Coulombe, K. L. K. (2019). Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomaterials Science & Engineering, 5, 887–899.
Kobayashi, K., Ichihara, Y., Tano, N., Fields, L., Murugesu, N., Ito, T., Ikebe, C., Lewis, F., Yashiro, K., Shintani, Y., Uppal, R., & Suzuki, K. (2018). Fibrin Glue-aided, Instant Epicardial Placement Enhances the Efficacy of Mesenchymal Stromal Cell-Based Therapy for Heart Failure. Scientific Reports, 8, 9448.
Gao, L., Kupfer, M. E., Jung, J. P., Yang, L., Zhang, P., Da Sie, Y., Tran, Q., Ajeti, V., Freeman, B. T., Fast, V. G., Campagnola, P. J., Ogle, B. M., & Zhang, J. (2017). Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circulation Research, 120, 1318–1325.
Shin, S. R., Zihlmann, C., Akbari, M., Assawes, P., Cheung, L., Zhang, K., Manoharan, V., Zhang, Y. S., Yuksekkaya, M., Wan, K. T., Nikkhah, M., Dokmeci, M. R., Tang, X. S., & Khademhosseini, A. (2016). Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small, 12, 3677–3689.
Noor, N., Shapira, A., Edri, R., Gal, I., Wertheim, L., & Dvir, T. (2019). 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv Sci (Weinh), 6, 1900344.
Miao, S., Cui, H., Nowicki, M., Lee, S. J., Almeida, J., Zhou, X., Zhu, W., Yao, X., Masood, F., Plesniak, M. W., Mohiuddin, M., & Zhang, L. G. (2018). Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation. Biofabrication, 10, 035007.
Li, Z., Fan, Z., Xu, Y., Lo, W., Wang, X., Niu, H., Li, X., Xie, X., Khan, M., & Guan, J. (2016). pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Applied Materials & Interfaces, 8, 10752–10760.
Wang, W., Tan, B., Chen, J., Bao, R., Zhang, X., Liang, S., Shang, Y., Liang, W., Cui, Y., Fan, G., Jia, H., & Liu, W. (2018). An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials, 160, 69–81.
Wanjare, M., Hou, L., Nakayama, K. H., Kim, J. J., Mezak, N. P., Abilez, O. J., Tzatzalos, E., Wu, J. C., & Huang, N. F. (2017). Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomaterials Science, 5, 1567–1578.
Pushp, P., Sahoo, B., Ferreira, F. C., Sampaio Cabral, J. M., Fernandes-Platzgummer, A., & Gupta, M. K. (2020). Functional comparison of beating cardiomyocytes differentiated from umbilical cord-derived mesenchymal/stromal stem cells and human foreskin-derived induced pluripotent stem cells. Journal of Biomedical Materials Research. Part A, 108, 496–514.
Pushp, P., Ferreira, F. C., Cabral, J. M. S., & Gupta, M. K. (2017). Improved survival of cardiac cells on surface modified electrospun nanofibers. Polymer Science, Series A, 59, 515–523.
Engelmayr Jr., G. C., Cheng, M., Bettinger, C. J., Borenstein, J. T., Langer, R., & Freed, L. E. (2008). Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Materials, 7, 1003–1010.
Radisic, M., Marsano, A., Maidhof, R., Wang, Y., & Vunjak-Novakovic, G. (2008). Cardiac tissue engineering using perfusion bioreactor systems. Nature Protocols, 3, 719–738.
Constantinides, C., Basnett, P., Lukasiewicz, B., Carnicer, R., Swider, E., Majid, Q. A., Srinivas, M., Carr, C. A., & Roy, I. (2018). In Vivo Tracking and (1)H/(19)F Magnetic Resonance Imaging of Biodegradable Polyhydroxyalkanoate/Polycaprolactone Blend Scaffolds Seeded with Labeled Cardiac Stem Cells. ACS Applied Materials & Interfaces, 10, 25056–25068.
Bertuoli, P. T., Ordono, J., Armelin, E., Perez-Amodio, S., Baldissera, A. F., Ferreira, C. A., Puiggali, J., Engel, E., Del Valle, L. J., & Aleman, C. (2019). Electrospun Conducting and Biocompatible Uniaxial and Core-Shell Fibers Having Poly(lactic acid), Poly(ethylene glycol), and Polyaniline for Cardiac Tissue Engineering. ACS Omega, 4, 3660–3672.
Hsiao, C. W., Bai, M. Y., Chang, Y., Chung, M. F., Lee, T. Y., Wu, C. T., Maiti, B., Liao, Z. X., Li, R. K., & Sung, H. W. (2013). Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials, 34, 1063–1072.
Stout, D. A., Yoo, J., Santiago-Miranda, A. N., & Webster, T. J. (2012). Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. International Journal of Nanomedicine, 7, 5653–5669.
Gelmi, A., Cieslar-Pobuda, A., de Muinck, E., Los, M., Rafat, M., & Jager, E. W. (2016). Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Advanced Healthcare Materials, 5, 1471–1480.
Khan, M., Xu, Y., Hua, S., Johnson, J., Belevych, A., Janssen, P. M., Gyorke, S., Guan, J., & Angelos, M. G. (2015). Evaluation of Changes in Morphology and Function of Human Induced Pluripotent Stem Cell Derived Cardiomyocytes (HiPSC-CMs) Cultured on an Aligned-Nanofiber Cardiac Patch. PLoS One, 10, e0126338.
Muniyandi, P., Palaninathan, V., Veeranarayanan, S., Ukai, T., Maekawa, T., Hanajiri, T., & Mohamed, M. S. (2020). ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering. Polymers (Basel), 12.
Mohammadi Amirabad, L., Massumi, M., Shamsara, M., Shabani, I., Amari, A., Mossahebi Mohammadi, M., Hosseinzadeh, S., Vakilian, S., Steinbach, S. K., Khorramizadeh, M. R., Soleimani, M., & Barzin, J. (2017). Enhanced Cardiac Differentiation of Human Cardiovascular Disease Patient-Specific Induced Pluripotent Stem Cells by Applying Unidirectional Electrical Pulses Using Aligned Electroactive Nanofibrous Scaffolds. ACS Applied Materials & Interfaces, 9, 6849–6864.
Boffito, M., Di Meglio, F., Mozetic, P., Giannitelli, S. M., Carmagnola, I., Castaldo, C., Nurzynska, D., Sacco, A. M., Miraglia, R., Montagnani, S., Vitale, N., Brancaccio, M., Tarone, G., Basoli, F., Rainer, A., Trombetta, M., Ciardelli, G., & Chiono, V. (2018). Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS One, 13, e0199896.
Gnanaprakasam Thankam, F., Muthu, J., Sankar, V., & Kozhiparambil Gopal, R. (2013). Growth and survival of cells in biosynthetic poly vinyl alcohol-alginate IPN hydrogels for cardiac applications. Colloids and Surfaces. B, Biointerfaces, 107, 137–145.
Pallavi, P., & Kumar, G. M. (2017). Synthesis and characterization of films based on cross linked blends of poly (vinylalcohol) and poly (vinylpyrrolidone) with glutaraldehyde for tissue engineering application: Synthese und Charakterisierung von Filmen auf der Basis von vernetzten Blends aus Poly (vinylalkohol) und Poly (vinylpyrrolidon) mit Glutaraldehyd zur Anwendung für Gewebeentwicklung. Materialwissenschaft und Werkstofftechnik, 48, 611–622.
Cui, Z., Ni, N. C., Wu, J., Du, G. Q., He, S., Yau, T. M., Weisel, R. D., Sung, H. W., & Li, R. K. (2018). Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics, 8, 2752–2764.
Pomeroy, J. E., Helfer, A., & Bursac, N. (2019). Biomaterializing the promise of cardiac tissue engineering. Biotechnology Advances.
Wang, L., Xu, C., Zhu, Y., Yu, Y., Sun, N., Zhang, X., Feng, K., & Qin, J. (2015). Human induced pluripotent stem cell-derived beating cardiac tissues on paper. Lab on a Chip, 15, 4283–4290.
Chun, Y. W., Balikov, D. A., Feaster, T. K., Williams, C. H., Sheng, C. C., Lee, J. B., Boire, T. C., Neely, M. D., Bellan, L. M., Ess, K. C., Bowman, A. B., Sung, H. J., & Hong, C. C. (2015). Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials, 67, 52–64.
Hou, L., Coller, J., Natu, V., Hastie, T. J., & Huang, N. F. (2016). Combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell-derived endothelial cells in hypoxia. Acta Biomaterialia, 44, 188–199.
Parveen, S., Singh, S. P., Panicker, M. M., & Gupta, P. K. (2019). Amniotic membrane as novel scaffold for human iPSC-derived cardiomyogenesis. In Vitro Cellular & Developmental Biology. Animal, 55, 272–284.
Arai, K., Murata, D., Verissimo, A. R., Mukae, Y., Itoh, M., Nakamura, A., Morita, S., & Nakayama, K. (2018). Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS One, 13, e0209162.
Tiburcy, M., Hudson, J. E., Balfanz, P., Schlick, S., Meyer, T., Chang Liao, M. L., Levent, E., Raad, F., Zeidler, S., Wingender, E., Riegler, J., Wang, M., Gold, J. D., Kehat, I., Wettwer, E., Ravens, U., Dierickx, P., van Laake, L. W., Goumans, M. J., Khadjeh, S., Toischer, K., Hasenfuss, G., Couture, L. A., Unger, A., Linke, W. A., Araki, T., Neel, B., Keller, G., Gepstein, L., Wu, J. C., & Zimmermann, W. H. (2017). Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation, 135, 1832–1847.
Oberwallner, B., Brodarac, A., Anić, P., Šarić, T., Wassilew, K., Neef, K., Choi, Y. H., & Stamm, C. (2015). Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. European Journal of Cardio-Thoracic Surgery, 47, 416–425 discussion 425.
Mayshar, Y., Ben-David, U., Lavon, N., Biancotti, J. C., Yakir, B., Clark, A. T., Plath, K., Lowry, W. E., & Benvenisty, N. (2010). Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell, 7, 521–531.
Mikkelsen, T. S., Hanna, J., Zhang, X., Ku, M., Wernig, M., Schorderet, P., Bernstein, B. E., Jaenisch, R., Lander, E. S., & Meissner, A. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature, 454, 49–55.
Kawamura, A., Miyagawa, S., Fukushima, S., Kawamura, T., Kashiyama, N., Ito, E., Watabe, T., Masuda, S., Toda, K., Hatazawa, J., Morii, E., & Sawa, Y. (2016). Teratocarcinomas Arising from Allogeneic Induced Pluripotent Stem Cell-Derived Cardiac Tissue Constructs Provoked Host Immune Rejection in Mice. Scientific Reports, 6, 19464.
Aron Badin, R., Bugi, A., Williams, S., Vadori, M., Michael, M., Jan, C., Nassi, A., Lecourtois, S., Blancher, A., Cozzi, E., Hantraye, P., & Perrier, A. L. (2019). MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates. Nature Communications, 10, 4357.
Zhao, T., Zhang, Z. N., Rong, Z., & Xu, Y. (2011). Immunogenicity of induced pluripotent stem cells. Nature, 474, 212–215.
de Almeida, P. E., Meyer, E. H., Kooreman, N. G., Diecke, S., Dey, D., Sanchez-Freire, V., Hu, S., Ebert, A., Odegaard, J., Mordwinkin, N. M., Brouwer, T. P., Lo, D., Montoro, D. T., Longaker, M. T., Negrin, R. S., & Wu, J. C. (2014). Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nature Communications, 5, 3903.
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A., & Bolton, E. M. (2012). Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell, 11, 147–152.
Liang, G., & Zhang, Y. (2013). Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell, 13, 149–159.
Kim, K., Doi, A., Wen, B., Ng, K., Zhao, R., Cahan, P., Kim, J., Aryee, M. J., Ji, H., Ehrlich, L. I., Yabuuchi, A., Takeuchi, A., Cunniff, K. C., Hongguang, H., McKinney-Freeman, S., Naveiras, O., Yoon, T. J., Irizarry, R. A., Jung, N., Seita, J., Hanna, J., Murakami, P., Jaenisch, R., Weissleder, R., Orkin, S. H., Weissman, I. L., Feinberg, A. P., & Daley, G. Q. (2010). Epigenetic memory in induced pluripotent stem cells. Nature, 467, 285–290.
Ohi, Y., Qin, H., Hong, C., Blouin, L., Polo, J. M., Guo, T., Qi, Z., Downey, S. L., Manos, P. D., Rossi, D. J., Yu, J., Hebrok, M., Hochedlinger, K., Costello, J. F., Song, J. S., & Ramalho-Santos, M. (2011). Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature Cell Biology, 13, 541–549.
Carcamo-Orive, I., Hoffman, G. E., Cundiff, P., Beckmann, N. D., D'Souza, S. L., Knowles, J. W., Patel, A., Papatsenko, D., Abbasi, F., Reaven, G. M., Whalen, S., Lee, P., Shahbazi, M., Henrion, M. Y. R., Zhu, K., Wang, S., Roussos, P., Schadt, E. E., Pandey, G., Chang, R., Quertermous, T., & Lemischka, I. (2017). Analysis of Transcriptional Variability in a Large Human iPSC Library Reveals Genetic and Non-genetic Determinants of Heterogeneity. Cell Stem Cell, 20, 518–532.e519.
Pei, F., Jiang, J., Bai, S., Cao, H., Tian, L., Zhao, Y., Yang, C., Dong, H., & Ma, Y. (2017). Chemical-defined and albumin-free generation of human atrial and ventricular myocytes from human pluripotent stem cells. Stem Cell Research, 19, 94–103.
Vitale, A. M., Matigian, N. A., Ravishankar, S., Bellette, B., Wood, S. A., Wolvetang, E. J., & Mackay-Sim, A. (2012). Variability in the generation of induced pluripotent stem cells: importance for disease modeling. Stem Cells Translational Medicine, 1, 641–650.
Volpato, V., & Webber, C. (2020). Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Disease Models & Mechanisms, 13, dmm042317.
Rojas, S. V., Martens, A., Zweigerdt, R., Baraki, H., Rathert, C., Schecker, N., Rojas-Hernandez, S., Schwanke, K., Martin, U., Haverich, A., & Kutschka, I. (2015). Transplantation Effectiveness of Induced Pluripotent Stem Cells Is Improved by a Fibrinogen Biomatrix in an Experimental Model of Ischemic Heart Failure. Tissue Engineering. Part A, 21, 1991–2000.
Rojas, S. V., Meier, M., Zweigerdt, R., Eckardt, D., Rathert, C., Schecker, N., Schmitto, J. D., Rojas-Hernandez, S., Martin, U., Kutschka, I., Haverich, A., & Martens, A. (2017). Multimodal Imaging for In Vivo Evaluation of Induced Pluripotent Stem Cells in a Murine Model of Heart Failure. Artificial Organs, 41, 192–199.
Martens, A., Rojas, S. V., Baraki, H., Rathert, C., Schecker, N., Zweigerdt, R., Schwanke, K., Rojas-Hernandez, S., Martin, U., Saito, S., Schmitto, J. D., Haverich, A., & Kutschka, I. (2014). Substantial early loss of induced pluripotent stem cells following transplantation in myocardial infarction. Artificial Organs, 38, 978–984.
Lou, X., Zhao, M., Fan, C., Fast, V. G., Valarmathi, M. T., Zhu, W., & Zhang, J. (2020). N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovascular Research, 116, 671–685.
Mauritz, C., Martens, A., Rojas, S. V., Schnick, T., Rathert, C., Schecker, N., Menke, S., Glage, S., Zweigerdt, R., Haverich, A., Martin, U., & Kutschka, I. (2011). Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. European Heart Journal, 32, 2634–2641.
Korecky, B., Hai, C. M., & Rakusan, K. (1982). Functional capillary density in normal and transplanted rat hearts. Canadian Journal of Physiology and Pharmacology, 60, 23–32.
Tee, R., Lokmic, Z., Morrison, W. A., & Dilley, R. J. (2010). Strategies in cardiac tissue engineering. ANZ Journal of Surgery, 80, 683–693.
Valarmathi, M. T., Fuseler, J. W., Davis, J. M., & Price, R. L. (2017). A Novel Human Tissue-Engineered 3-D Functional Vascularized Cardiac Muscle Construct. Frontiers in Cell and Development Biology, 5, 2.
Acknowledgments
Support (Project ID: 1-5763895621) from the Technical Education Quality Improvement Program (TEQIP-III), implemented through the Ministry of Human Resource Development – National Project Implementation Unit (MHRD-NPIU) is acknowledged. The support of Erasmus Mundus (NAMASTE) for exchange mobility of Pallavi Pushp and Erasmus+ for exchange mobility of Mukesh Gupta to Institute Superior Tecnico (IST), Portugal is also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pushp, P., Nogueira, D.E.S., Rodrigues, C.A.V. et al. A Concise Review on Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Personalized Regenerative Medicine. Stem Cell Rev and Rep 17, 748–776 (2021). https://doi.org/10.1007/s12015-020-10061-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10061-2