Abstract
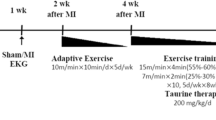
The formation of new blood vessels in the ischemic area is a fundamental strategy that can reduce myocardial infarction-induced damage by mitigating hypoxia. This paper set out to investigate the cardioprotective effect of high-intensity interval training preconditioning and L-arginine supplementation on myocardial ischemia–reperfusion-induced angiogenesis and oxidative stress. 50 male rats were randomly distributed into following groups: (1) Sham, (2) Sedentary control (Con, n = 10), 3) L-arginine treatment (La, n = 10), (4) High-Intensity Interval Training (HIIT, n = 10), and High-Intensity Interval Training plus L-arginine supplementation (HIIT + La, n = 10). Rats in the training groups performed high-intensity interval training for 8 weeks (5 day per week). Subjects in La and HIIT + La groups received L-arginine in drinking water (4 g/L). 72 h after treatments, all subjects underwent myocardial ischemia–reperfusion operation. Cardiac function, angiogenesis, stress oxidative, and infarction size were measured after reperfusion. Results showed exercise training and L-arginine supplementation promoted Cat and GSH activities and decreased MDA activity following myocardial ischemia–reperfusion injury in non-infarcted area. Compared with the con group, VEGF and Ang-1 as well as Ang-1 to Ang-2 ratio following myocardial ischemia–reperfusion in the non-infarct area were higher in La + HIIT group. Meanwhile, capillary density and capillary-to-muscle fiber ratio were higher in response to training and L-arginine supplementation. HIIT and L-arginine alone and synergistically decreased ischemia–reperfusion-induced infarction size. Cardiac output and stroke volume ameliorate in response to exercise training and L-arginine supplementation. Taken together, exercise preconditioning and l-arginine supplementation improved left ventricular function following ischemia–reperfusion by stress oxidative mitigation and angiogenesis amelioration.


Similar content being viewed by others
Data Availability
The dataset used and analyzed during the current study is available from Dr. Ranjbar on reasonable request.
Code Availability
Not applicable.
References
Kim, Y.-W., & Byzova, T. V. (2014). Oxidative stress in angiogenesis and vascular disease. Blood, the Journal of the American Society of Hematology, 123(5), 625–631.
Banaei, P., et al. (2020). Preconditioning effect of high-intensity interval training (HIIT) and berberine supplementation on the gene expression of angiogenesis regulators and caspase-3 protein in the rats with myocardial ischemia-reperfusion (IR) injury. BioMed Research International, 2020, 1–9.
Veeranki, S., & Tyagi, S. C. (2017). Mdivi-1 induced acute changes in the angiogenic profile after ischemia-reperfusion injury in female mice. Physiological reports, 5(11), e13298.
Yamauchi, A., et al. (2003). Pre-administration of angiopoietin-1 followed by VEGF induces functional and mature vascular formation in a rabbit ischemic model. The Journal of Gene Medicine: A cross-disciplinary journal for research on the science of gene transfer and its clinical applications, 5(11), 994–1004.
Fagiani, E., & Christofori, G. (2013). Angiopoietins in angiogenesis. Cancer letters, 328(1), 18–26.
Shim, W. S., et al. (2006). Angiopoietin-1 promotes functional neovascularization that relieves ischemia by improving regional reperfusion in a swine chronic myocardial ischemia model. Journal of biomedical science, 13(4), 579–591.
Zan, L., et al. (2011). Expression and function of vascular endothelial growth factor and angiopoietins in rat brain after cerebral ischemia. Zhonghua bing li xue za zhi = Chinese Journal of Pathology, 40(12), 834–839.
Shyu, K.-G., et al. (2003). Increased expression of angiopoietin-2 and Tie2 receptor in a rat model of myocardial ischaemia/reperfusion. Clinical science, 105(3), 287–294.
Ray, P. S., et al. (2000). Early effects of hypoxia/reoxygenation on VEGF, ang-1, ang-2 and their receptors in the rat myocardium: Implications for myocardial angiogenesis. Molecular and Cellular Biochemistry, 213(1), 145–153.
Weyrich, A. S., Ma, X.-L., & Lefer, A. M. (1992). The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation, 86(1), 279–288.
Zheng, H., et al. (1999). Effects of myocardial ischemia reperfusion injury on L-arginine/nitric oxide system in rat heart. Sheng li xue bao: [Acta physiologica Sinica], 51(1), 25–30.
Chander, V., & Chopra, K. (2005). Role of nitric oxide in resveratrol-induced renal protective effects of ischemic preconditioning. Journal of vascular surgery, 42(6), 1198–1205.
Luo, Z., et al. (1997). Vascular endothelial growth factor attenuates myocardial ischemia-reperfusion injury. The Annals of thoracic surgery, 64(4), 993–998.
Khalafi, M., et al. (2020). The impact of moderate-intensity continuous or high-intensity interval training on adipogenesis and browning of subcutaneous adipose tissue in obese male rats. Nutrients, 12(4), 925.
Ranjbar, K., Nazem, F., & Nazari, A. (2016). Effect of exercise training and L-arginine on oxidative stress and left ventricular function in the post-ischemic failing rat heart. Cardiovascular toxicology, 16(2), 122–129.
Ranjbar, K., et al. (2018). Cardioprotective effect of resistance training and Crataegus oxyacantha extract on ischemia reperfusion–induced oxidative stress in diabetic rats. Biomedicine & Pharmacotherapy, 100, 455–460.
La Bonte, L. R., et al. (2008). Complement inhibition reduces injury in the type 2 diabetic heart following ischemia and reperfusion. American Journal of Physiology-Heart and Circulatory Physiology, 294(3), H1282–H1290.
Powers, S. K., Quindry, J. C., & Kavazis, A. N. (2008). Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radical Biology and Medicine, 44(2), 193–201.
Quindry, J., et al. (2005). Exercise training provides cardioprotection against ischemia–reperfusion induced apoptosis in young and old animals. Experimental Gerontology, 40(5), 416–425.
Ranjbar, K., et al., Synergistic effects of nitric oxide and exercise on revascularisation in the infarcted ventricle in a murine model of myocardial infarction.
Zhang, Y., et al. (2010). Tanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes, Obesity and Metabolism, 12(4), 316–322.
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine, 70(1), 158–169.
Sedlak, J., & Lindsay, R. H. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical biochemistry, 25, 192–205.
Aebi, H. (1984). [13] Catalase in vitro. Methods in Enzymology, 105, 121–126.
Esterbauer, H., & Zollern, H. (1989). Methods for determination of aldehydic lipid peroxidation products. Free Radical Biology and Medicine, 7(2), 197–203.
Ahmadvand, H., et al. (2017). Protective effects of oleuropein against renal injury oxidative damage in alloxan-induced diabetic rats; a histological and biochemical study. Journal of nephropathology, 6(3), 204.
Ramez, M., et al. (2020). High-intensity interval training increases myocardial levels of Klotho and protects the heart against ischaemia–reperfusion injury. Experimental physiology, 105(4), 652–665.
Ustunova, S., et al. (2020). Hydrogen sulphide and nitric oxide cooperate in cardioprotection against ischemia/reperfusion injury in isolated rat heart. In Vivo, 34(5), 2507–2516.
Weerateerangkul, P., Chattipakorn, S., & Chattipakorn, N. (2011). Roles of the nitric oxide signaling pathway in cardiac ischemic preconditioning against myocardial ischemia-reperfusion injury. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 17(2), RA44.
Fallahi, A., et al. (2015). Cardioprotective effect of high intensity interval training and nitric oxide metabolites (NO2−, NO3−). Iranian Journal of Public Health, 44(9), 1270.
Brüne, B., von Knethen, A., & Sandau, K. B. (1998). Nitric oxide and its role in apoptosis. European Journal of Pharmacology, 351(3), 261–272.
Huang, S., et al. (2021). Co-expression of tissue kallikrein 1 and tissue inhibitor of matrix metalloproteinase 1 improves myocardial ischemia-reperfusion injury by promoting angiogenesis and inhibiting oxidative stress. Molecular Medicine Reports, 23(2), 1–1.
Xian, D., et al. (2019). Emerging roles of redox-mediated angiogenesis and oxidative stress in dermatoses. Oxidative Medicine and Cellular Longevity, 2019, 1–14.
Matsunaga, T., et al. (2003). Expression of VEGF and angiopoietins-1 and-2 during ischemia-induced coronary angiogenesis. American Journal of Physiology-Heart and Circulatory Physiology, 285(1), H352–H358.
Dallabrida, S. M., et al. (2005). Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circulation Research, 96(4), e8–e24.
Lin, W.-T., et al. (2006). L-Arginine attenuates xanthine oxidase and myeloperoxidase activities in hearts of rats during exhaustive exercise. British Journal of Nutrition, 95(1), 67–75.
Ranjbar, K., Nazem, F., & Nazari, A. (2015). Effect of exercise training and L-arginine on oxidative stress and left ventricular function in the post-ischemic failing rat heart. Cardiovascular Toxicology, 16, 122–129.
Ohta, Y., & Nishida, K. (2001). Protective effect of L-arginine against stress-induced gastric mucosal lesions in rats and its relation to nitric oxide-mediated inhibition of neutrophil infiltration. Pharmacological Research, 43(6), 535–541.
Duzova, H., et al. (2009). Effects of acute moderate and strenuous exercise bouts on IL-17 production and inflammatory response in trained rats. Journal of Sports Science & Medicine, 8(2), 219.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The experimental protocols were in accordance with the ethical standards and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and this study was approved by ethics committee of Lorestan University of medical science.
Consent for Publication
The author is ready to publish the manuscript in ‘Cardiovascular Toxicology’ as per rules and regulations of the journal.
Consent to Participate
Not applicable.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjbar, K. Improved Cardiac Function Following Ischemia Reperfusion Injury Using Exercise Preconditioning and L-Arginine Supplementation via Oxidative Stress Mitigation and Angiogenesis Amelioration. Cardiovasc Toxicol 22, 736–745 (2022). https://doi.org/10.1007/s12012-022-09752-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09752-8