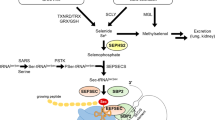
Abstract
Selenium plays a crucial role as a micronutrient, primarily exerting its biological functions through selenoproteins. It has been established that selenium deficiency adversely impacts cartilage development, leading to alterations in chondrocyte function. In regions with low selenium intake, endemic osteochondrosis has been documented, characterized by compromised growth plate and articular cartilage formation. Vascular endothelial growth factor (VEGF) stands out as a pivotal angiogenic factor, with elevated levels contributing significantly to vascular invasion into chondrocytes. This VEGF-mediated invasion serves as a key signal, prompting morphological changes in the growth plate and initiating cartilage remodeling. In animal models, the selenium deficiency group exhibited heightened levels of the cartilage damage marker matrix metalloproteinases 13 (MMP13). This resulted in articular cartilage degeneration, accompanied by a substantial increase in VEGF expression within the growth plate and articular cartilage, as compared to the normal group. In a chondrogenic progenitor cell (CPC) differentiation model, insufficient selenium induced chondrocyte damage and upregulated inflammatory factors such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX2). The selenium-deficient groups showed elevated expressions of VEGF, VEGFR2, MMP13, Collagen X, and Angiopoietin 1, accelerating the degradation of the extracellular matrix (ECM), which further promoted the development of cartilage-related diseases. Taken together, these findings provide novel insights for a better understanding of the role of low selenium in cartilage degeneration and angiogenesis. They shed light on the intricate influence of low selenium levels on the development of articular cartilage, emphasizing the interconnected pathways and processes involved.





Similar content being viewed by others
Data Availability
The data generated in this study are available from the corresponding author upon reasonable request.
References
Yan J, Zheng Y, Min Z, Ning Q, Lu S (2013) Selenium effect on selenoprotein transcriptome in chondrocytes. Biometals 26(2):285–296
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity, Nutrients 10(9)
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94(3):739–777
Schomburg L (2011) Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 8(3):160–171
Guillin OM, Vindry C, Ohlmann T, Chavatte L (2019) Selenium, selenoproteins and viral infection, Nutrients 11(9)
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268
Kang D, Lee J, Wu C, Guo X, Lee BJ, Chun JS, Kim JH (2020) The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med 52(8):1198–1208
Donadio JLS, Duarte GBS, Borel P, Cozzolino SMF, Rogero MM (2021) The influence of nutrigenetics on biomarkers of selenium nutritional status. Nutr Rev 79(11):1259–1273
Yan J, Tian J, Zheng Y, Han Y, Lu S (2012) Selenium promotes proliferation of chondrogenic cell ATDC5 by increment of intracellular ATP content under serum deprivation. Cell Biochem Funct 30(8):657–663
Yan J, Xu J, Fei Y, Jiang C, Zhu W, Han Y, Lu S (2016) TrxR2 deficiencies promote chondrogenic differentiation and induce apoptosis of chondrocytes through mitochondrial reactive oxygen species. Exp Cell Res 344(1):67–75
Zhang F, Wu C, Zhang P, Wang X, Meng P, Tan S, Yuan L, Guo X (2022) Abnormal level of manganese, iron, iodine, and selenium in the hair of children living in Kashin-Beck disease endemic areas. Biol Trace Elem Res 200(10):4278–4288
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP (2016) Osteoarthritis. Nat Rev Dis Primers 2:16072
Min Z, Zhao W, Zhong N, Guo Y, Sun M, Wang Q, Zhang R, Yan J, Tian L, Zhang F, Han Y, Ning Q, Meng L, Sun J, Lu S (2015) Abnormality of epiphyseal plate induced by selenium deficiency diet in two generation DA rats. APMIS 123(8):697–705
Wang L, Yin J, Yang B, Qu C, Lei J, Han J, Guo X (2020) Serious selenium deficiency in the serum of patients with Kashin-Beck disease and the effect of nano-selenium on their chondrocytes. Biol Trace Elem Res 194(1):96–104
Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R (2004) Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol 202(3):367–374
Nagao M, Hamilton JL, Kc R, Berendsen AD, Duan X, Cheong CW, Li X, Im HJ, Olsen BR (2017) Vascular endothelial growth factor in cartilage development and osteoarthritis. Sci Rep 7(1):13027
Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS (2001) Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15(21):2865–2876
Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH (2005) Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cells Mater 9:58–67 (discussion 67)
Yan J, Fei Y, Han Y, Lu S (2016) Selenoprotein O deficiencies suppress chondrogenic differentiation of ATDC5 cells. Cell Biol Int 40(10):1033–1040
Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N (2009) Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4(4):324–335
Wang L, Guo X, Yi J, Qu C, Lei J, Guo Q, Han J (2018) The effects of long-term low selenium diet on the expression of CHST-3, CHST-12 and UST in knee cartilage of growing rats. J Trace Elem Med Biol 50:123–129
Han J, Liang H, Yi J, Tan W, He S, Wu X, Shi X, Ma J, Guo X (2016) Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J Trace Elem Med Biol 34:1–9
Han J, Liang H, Yi J, Tan W, He S, Wang S, Li F, Wu X, Ma J, Shi X, Guo X, Bai C (2017) Long-term selenium-deficient diet induces liver damage by altering hepatocyte ultrastructure and MMP1/3 and TIMP1/3 expression in growing rats. Biol Trace Elem Res 175(2):396–404
Kim HK, Bian H, Randall T, Garces A, Gerstenfeld LC, Einhorn TA (2004) Increased VEGF expression in the epiphyseal cartilage after ischemic necrosis of the capital femoral epiphysis. J Bone Miner Res 19(12):2041–2048
Cheung WH, Lee KM, Fung KP, Leung KS (2001) Growth plate chondrocytes inhibit neo-angiogenesis – a possible mechanism for tumor control. Cancer Lett 163(1):25–32
Guo X, Zuo H, Cao CX, Zhang Y, Geng D, Zhang ZT, Zhang YG, von der Mark K, von der Mark H (2006) Abnormal expression of Col X, PTHrP, TGF-beta, bFGF, and VEGF in cartilage with Kashin-Beck disease. J Bone Miner Metab 24(4):319–328
Wang H, Li Z, Liu Y, Zhang M, Shi Y, Zhang Y, Mi G, Wang M, He Y, Chen Y, Chen C, Chen J (2023) Effects of selenoprotein s knockdown on endoplasmic reticulum stress in ATDC5 cells and gene expression profiles in hypertrophic chondrocytes. Biol Trace Elem Res 201(4):1965–1976
Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B (2003) Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol 162(5):833–842
Gao ZQ, Guo X, Duan C, Ma W, Xu P, Wang W, Chen JC (2012) Altered aggrecan synthesis and collagen expression profiles in chondrocytes from patients with Kashin-Beck disease and osteoarthritis. J Int Med Res 40(4):1325–1334
Guan F, Li S, Wang ZL, Yang H, Xue S, Wang W, Song D, Zhou X, Zhou W, Chen JH, Caterson B, Hughes C (2013) Histopathology of chondronecrosis development in knee articular cartilage in a rat model of Kashin-Beck disease using T-2 toxin and selenium deficiency conditions. Rheumatol Int 33(1):157–166
Streicher KL, Sylte MJ, Johnson SE, Sordillo LM (2004) Thioredoxin reductase regulates angiogenesis by increasing endothelial cell-derived vascular endothelial growth factor. Nutr Cancer 50(2):221–231
Pufe T, Kurz B, Petersen W, Varoga D, Mentlein R, Kulow S, Lemke A, Tillmann B (2005) The influence of biomechanical parameters on the expression of VEGF and endostatin in the bone and joint system. Ann Anat 187(5–6):461–472
Lingaraj K, Poh CK, Wang W (2010) Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann Acad Med Singap 39(5):399–403
Beckmann R, Houben A, Tohidnezhad M, Kweider N, Fragoulis A, Wruck CJ, Brandenburg LO, Hermanns-Sachweh B, Goldring MB, Pufe T, Jahr H (2014) Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. Int J Mol Sci 15(9):15456–15474
Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, Malaise M, de Seny D (2019) Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol 165:49–65
Acknowledgements
We thank all individuals who participated in this work.
Funding
This study was supported by grants from the National Science Foundation of China (81672769).
Author information
Authors and Affiliations
Contributions
Qing Bi and Qiong Zhang were responsible for supervision, validation, and funding acquisition. Xiang Meng was responsible for the verification experiment and writing—original draft preparation. Xiumei Meng was responsible for writing and editing. Ye Yuan, Zeju He, Yong Fan, Li Yin, Yu Tong, Zheping Hong, and Senbo Zhu contributed to the manuscript revision, searching the literature, and reading the submitted version. All authors contributed to the review and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Medical Animal Research Ethics Committee of Zhejiang Provincial People’s Hospital (approval number: A2022060101).
Conflict of Interest
The authors declare no competing interests.
Consent for Publication
We have obtained consent to publish this paper from all the participants of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, X., Meng, X., He, Z. et al. Selenium Deficiency Can Promote the Expression of VEGF and Inflammatory Factors in Cartilage Differentiation and Mediates Cartilage Injury. Biol Trace Elem Res (2023). https://doi.org/10.1007/s12011-023-04003-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-023-04003-5