Abstract
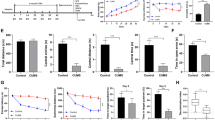
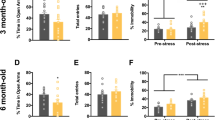
Selenium binding protein 1 (SELENBP1) is involved in neurologic disorders, such as multiple sclerosis, spinal cord injury, Parkinson’s disease, epilepsy, and schizophrenia. However, the role of SELENBP1 in the neurogenesis of depression, which is a neurologic disorder, and the underlying mechanisms of oxidative stress and inflammation in depression remain unknown. In this study, we evaluated the changes in the expression levels of SELENBP1 in the hippocampus of a mouse model of depression and in the serum of human patients with depression using the Gene Expression Omnibus database. These changes were validated using blood samples from human patients with depression and mouse models with chronic unpredictable mild stress (CUMS)-induced depressive-like behavior. We also investigated the effects of SELENBP1 knockout (KO) on inflammation, oxidative stress, and hippocampal neurogenesis in mice with CUMS-induced depression. Our results revealed that SELENBP1 levels was decreased in the blood of human patients with depression and in the hippocampus of mice with CUMS-induced depression. SELENBP1 KO increased CUMS-induced depressive behavior in mice and caused dysregulation of inflammatory cytokines and oxidative stress. This led to a decrease in the numbers of doublecortin- and Ki67-positive cells, which might aggravate CUMS-induced depressive symptoms. These findings suggest that SELENBP1 might be involved in the regulation of neurogenesis in mice with depression and could be served as a potential target for diagnosing and treating depression.
Graphical Abstract








Similar content being viewed by others
Data Availability
Data will be made available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- AU:
-
Arbitrary unit
- CAT:
-
Catalase
- CUMS:
-
Chronic unpredictable mild stress
- DCX:
-
Doublecortin
- DG:
-
Dentate gyrus
- GPX:
-
Glutathione peroxidase
- IL-10:
-
Interleukin 10
- IL-1β:
-
Interleukin 1β
- KO:
-
Knockout
- MDA:
-
Malondialdehyde
- ns:
-
No significance
- PD:
-
Parkinson's disease
- ROS:
-
Reactive oxygen species
- SELENBP1:
-
Selenium binding protein 1
- SELENOK:
-
Selenoprotein K
- SELENOP:
-
Selenoprotein P
- SELENOT:
-
Selenoprotein T
- SELENOW:
-
Selenoprotein W
- TGF-β:
-
Transforming growth factor β
- TNF-α:
-
Tumor necrosis factor-α
- WT:
-
Wild type
References
Pillai R, Uyehara-Lock JH, Bellinger FP (2014) Selenium and selenoprotein function in brain disorders. IUBMB Life 66(4):229–239
Cardoso BR, Roberts BR, Bush AI, Hare DJ (2015) Selenium, selenoproteins and neurodegenerative diseases. Metallomics 7(8):1213–1228
Zhang ZH, Wu QY, Chen C, Zheng R, Chen Y, Ni JZ, Song GL (2018) Comparison of the effects of selenomethionine and selenium-enriched yeast in the triple-transgenic mouse model of Alzheimer’s disease. Food Funct 9(7):3965–3973
Zhang ZH, Cao XC, Peng JY, Huang SL, Chen C, Jia SZ, Ni JZ, Song GL (2022) Reversal of lipid metabolism dysregulation by selenium and folic acid co-supplementation to mitigate pathology in Alzheimer’s disease. Antioxidants 11(5):829
Zhang ZH, Chen C, Jia SZ, Cao XC, Liu M, Tian J, Hoffmann PR, Xu HX, Ni JZ, Song GL (2021) Selenium restores synaptic deficits by modulating NMDA receptors and selenoprotein K in an Alzheimer’s disease model. Antioxid Redox Signal 35(11):863–884
Chen C, Chen Y, Zhang ZH, Jia SZ, Chen YB, Huang SL, Xu XW, Song GL (2021) Selenomethionine improves mitochondrial function by upregulating mitochondrial selenoprotein in a model of Alzheimer’s disease. Front Aging Neurosci 13:750921
Li X, Shi Q, Xu H, Xiong Y, Wang C, Le L, Lian J, Wu G, Peng F, Liu Q, Du X (2022) Ebselen interferes with Alzheimer’s disease by regulating mitochondrial function. Antioxidants 11(7):1350
Zhang ZH, Song GL (2021) Roles of selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Front Neurosci 15:646518
Du X, Wang C, Liu Q (2016) Potential roles of selenium and selenoproteins in the prevention of Alzheimer’s disease. Curr Top Med Chem 16(8):835–848
Solovyev N, Drobyshev E, Bjørklund G, Dubrovskii Y, Lysiuk R, Rayman MP (2018) Selenium, selenoprotein P, and Alzheimer’s disease: is there a link? Free Radic Biol Med 127:124–133
Solovyev N (2020) Selenoprotein P and its potential role in Alzheimer’s disease. Hormones 19(1):73–79
Achilli C, Ciana A, Minetti G (2018) Brain, immune system and selenium: a starting point for a new diagnostic marker for Alzheimer's disease?. Perspect Public Health 138(4):223–226
Zhang X, Ye YL, Zhu H, Sun SN, Zheng J, Fan HH, Wu HM, Chen SF, Cheng WH, Zhu JH (2016) Selenotranscriptomic analyses identify signature selenoproteins in brain regions in a mouse model of Parkinson’s disease. PLoS One 11(9):e0163372
Zhang L, Zhu JH, Zhang X, Cheng WH (2019) The thioredoxin-like family of selenoproteins: implications in aging and age-related degeneration. Biol Trace Elem Res 188(1):189–195
Boukhzar L, Hamieh A, Cartier D, Tanguy Y, Alsharif I, Castex M, Arabo A, El Hajji S, Bonnet JJ, Errami M, Falluel-Morel A, Chagraoui A, Lihrmann I, Anouar Y (2016) Selenoprotein T exerts an essential oxidoreductase activity that protects dopaminergic neurons in mouse models of Parkinson’s disease. Antioxid Redox Signal 24(11):557–574
Shao ZQ, Zhang X, Fan HH, Wang XS, Wu HM, Zhang L, Cheng WH, Zhu JH (2019) Selenoprotein T promotes proliferation and G1-to-S transition in SK-N-SH cells: implications in Parkinson’s disease. J Nutr 149(12):2110–2119
Zhang X, Liu RP, Cheng WH, Zhu JH (2019) Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech Ageing Dev 180:89–96
Stefaniuk M, Lukasiuk K (2010) Cloning of expressed sequence tags (ESTs) representing putative epileptogenesis-related genes and the localization of their expression in the normal brain. Neurosci Lett 482(3):230–234
Yüzbaşioğlu A, Karataş H, Gürsoy-Ozdemir Y, Saygi S, Akalan N, Söylemezoğlu F, Dalkara T, Kocaefe YC, Ozgüç M (2009) Changes in the expression of selenoproteins in mesial temporal lobe epilepsy patients. Cell Mol Neurobiol 29(8):1223–1231
Zhang S, Rocourt C, Cheng WH (2010) Selenoproteins and the aging brain. Mech Ageing Dev 131(4):253–260
Ferreira de Almeida TL, Petarli GB, Cattafesta M, Zandonade E, Bezerra OMPA, Tristão KG, Salaroli LB (2021) Association of selenium intake and development of depression in Brazilian farmers. Front Nutr 8:671377
Sajjadi SS, Foshati S, Haddadian-Khouzani S, Rouhani MH (2022) The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Rep 12(1):1045
Samad N, Rao T, Rehman MHU, Bhatti SA, Imran I (2022) Inhibitory effects of selenium on arsenic-induced anxiety-/depression-like behavior and memory impairment. Biol Trace Elem Res 200(2):689–698
Jia SZ, Xu XW, Zhang ZH, Chen C, Chen YB, Huang SL, Liu Q, Hoffmann PR, Song GL (2021) Selenoprotein K deficiency-induced apoptosis: A role for calpain and the ERS pathway. Redox Biol 47:102154
Situ J, Huang X, Zuo M, Huang Y, Ren B, Liu Q (2022) Comparative proteomic analysis reveals the effect of selenoprotein W deficiency on oligodendrogenesis in fear memory. Antioxidants 11(5):999
Sasuclark AR, Khadka VS, Pitts MW (2019) Cell-type specific analysis of selenium-related genes in brain. Antioxidants 8(5):120
Jia Y, Dai J, Zhang L, Xia H (2019) Biological functions of selenium-binding protein 1 and its relationship with diseases. Prog Biochem Biophys 46(2):128–137
Elhodaky M, Diamond AM (2018) Selenium-binding protein 1 in human health and disease. Int J Mol Sci 19(11):3437
Elkjaer ML, Nawrocki A, Kacprowski T, Lassen P, Simonsen AH, Marignier R, Sejbaek T, Nielsen HH, Wermuth L, Rashid AY, Høgh P, Sellebjerg F, Reynolds R, Baumbach J, Larsen MR, Illes Z (2021) CSF proteome in multiple sclerosis subtypes related to brain lesion transcriptomes. Sci Rep 11(1):4132
Heller RA, Seelig J, Crowell HL, Pilz M, Haubruck P, Sun Q, Schomburg L, Daniel V, Moghaddam A, Biglari B (2021) Predicting neurological recovery after traumatic spinal cord injury by time-resolved analysis of monocyte subsets. Brain 144(10):3159–3174
Seelig J, Heller RA, Haubruck P, Sun Q, Georg Klingenberg J, Hackler J, Crowell HL, Daniel V, Moghaddam A, Schomburg L, Biglari B (2021) Selenium-binding protein 1 (SELENBP1) as biomarker for adverse clinical outcome after traumatic spinal cord injury. Front Neurosci 15:680240
Gu Y, Wu H, Wang T, Yu S, Han Z, Zhang W, Mu L, Wang H, Na M, Wang H, Lin Z (2022) Profiling analysis of circular RNA and mRNA in human temporal lobe epilepsy with hippocampal sclerosis ILAE type 1. Cell Mol Neurobiol 42(8):2745–2755
Mohammadi A, Rashidi E, Amooeian VG (2018) Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatry Res 265:25–38
Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sásik R, Khanlou N, Han M, Liew CC, Tsuang MT (2005) Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci 102(43):15533–15538
Udawela M, Money TT, Neo J, Seo MS, Scarr E, Dean B, Everall IP (2015) SELENBP1 expression in the prefrontal cortex of subjects with schizophrenia. Transl Psychiatry 5(8):e615
Ishida YI, Kayama T, Kibune Y, Nishimoto S, Koike S, Suzuki T, Horiuchi Y, Miyashita M, Itokawa M, Arai M, Ogasawara Y (2017) Identification of an argpyrimidine-modified protein in human red blood cells from schizophrenic patients: A possible biomarker for diseases involving carbonyl stress. Biochem Biophys Res Commun 493(1):573–577
Chau EJ, Mostaid MS, Cropley V, McGorry P, Pantelis C, Bousman CA, Everall IP (2018) Downregulation of plasma SELENBP1 protein in patients with recent-onset schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 85:1–6
Wang H, Jin M, Xie M, Yang Y, Xue F, Li W, Zhang M, Li Z, Li X, Jia N, Liu Y, Cui X, Hu G, Dong L, Wang G, Yu Q (2023) Protective role of antioxidant supplementation for depression and anxiety: A meta-analysis of randomized clinical trials. J Affect Disord 323:264–279
Bhatt S, Nagappa AN, Patil CR (2020) Role of oxidative stress in depression. Drug Discov Today 25(7):1270–1276
Bhatt S, Devadoss T, Jha NK, Baidya M, Gupta G, Chellappan DK, Singh SK, Dua K (2023) Targeting inflammation: a potential approach for the treatment of depression. Metab Brain Dis 38(1):45–59
Zuo C, Cao H, Song Y, Gu Z, Huang Y, Yang Y, Miao J, Zhu L, Chen J, Jiang Y, Wang F (2022) Nrf2: An all-rounder in depression. Redox Biol 58:102522
Liu CS, Adibfar A, Herrmann N, Gallagher D, Lanctôt KL (2017) Evidence for inflammation-associated depression. Curr Top Behav Neurosci 31:3–30
Gałecki P, Talarowska M (2018) Inflammatory theory of depression. Psychiatr Pol 52(3):437–447
Albrakati A, Alsharif KF, Al Omairi NE, Alsanie WF, Almalki ASA, Abd Elmageed ZY, Elshopakey GE, Lokman MS, Bauomy AA, Abdel Moneim AE, Kassab RB (2021) Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. Int J Nanomedicine 16:8447–8464
Bai J, Yu J, Wang J, Xue B, He N, Tian Y, Yang L, Wang Y, Wang Y, Tang Q (2019) DNA methylation of miR-122 aggravates oxidative stress in colitis targeting SELENBP1 partially by p65NF-κB signaling. Oxid Med Cell Longev 2019:5294105
Correia AS, Cardoso A, Vale N (2023) Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 12(2):470
Sánchez-Vidaña DI, Po KK, Fung TK, Chow JK, Lau WK, So PK, Lau BW, Tsang HW (2019) Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats. Neurosci Lett 701:180–192
Fatima M, Srivastav S, Ahmad MH, Mondal AC (2019) Effects of chronic unpredictable mild stress induced prenatal stress on neurodevelopment of neonates: Role of GSK-3β. Sci Rep 9(1):1305
Jiang N, Huang H, Zhang Y, Lv J, Wang Q, He Q, Liu X (2021) Ginsenoside Rb1 produces antidepressant-like effects in a chronic social defeat stress model of depression through the BDNF-Trkb signaling pathway. Front Pharmacol 12:680903
Zhang K, Pan X, Wang F, Ma J, Su G, Dong Y, Yang J, Wu C (2016) Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci Rep 6:30951
Ma ZX, Zhang RY, Rui WJ, Wang ZQ, Feng X (2021) Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway. Behav Brain Res 406:113245
Leiter O, Zhuo Z, Rust R, Wasielewska JM, Grönnert L, Kowal S, Overall RW, Adusumilli VS, Blackmore DG, Southon A, Ganio K, McDevitt CA, Rund N, Brici D, Mudiyan IA, Sykes AM, Rünker AE, Zocher S, Ayton S, Bush AI, Bartlett PF, Hou ST, Kempermann G, Walker TL (2022) Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab 34(3):408-423.e8
Peng YL, Liu YN, Liu L, Wang X, Jiang CL, Wang YX (2012) Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflammation 9:75
Li Y, Wang L, Wang P, Fan C, Zhang P, Shen J, Yu SY (2020) Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats. Oxid Med Cell Longev 2020:2325391
Fan C, Long Y, Wang L, Liu X, Liu Z, Lan T, Li Y, Yu SY (2020) N-acetylcysteine rescues hippocampal oxidative stress-induced neuronal injury via suppression of p38/JNK signaling in depressed rats. Front Cell Neurosci 14:554613
Li K, Yan L, Zhang Y, Yang Z, Zhang C, Li Y, Kalueff AV, Li W, Song C (2020) Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J Ethnopharmacol 250:112487
Xia H, Wang Y, Dai J, Zhang X, Zhou J, Zeng Z, Jia Y (2022) Selenoprotein K is essential for the migration and phagocytosis of immature dendritic cells. Antioxidants 11(7):1264
Zhang C, Xu W, Pan W, Wang N, Li G, Fan X, Xu X, Shen S, Das UN (2013) Selenium-binding protein 1 may decrease gastric cellular proliferation and migration. Int J Oncol 42(5):1620–1629
Steinbrenner H, Micoogullari M, Hoang NA, Bergheim I, Klotz LO, Sies H (2019) Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol 20:489–495
Zafar S, Noor A, Younas N, Shafiq M, Schmitz M, Wurster I, Brockmann K, Gasser T, Zerr I (2022) SWATH mass spectrometry-based CSF proteome profile of GBA-linked Parkinson’s disease patients. Int J Mol Sci 23(22):14166
Tsujimoto S, Ishida T, Takeda T, Ishii Y, Onomura Y, Tsukimori K, Takechi S, Yamaguchi T, Uchi H, Suzuki SO, Yamamoto M, Himeno M, Furue M (1830) Yamada H (2013) Selenium-binding protein 1: its physiological function, dependence on aryl hydrocarbon receptors, and role in wasting syndrome by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Biophys Acta 6:3616–3624
Belleau EL, Treadway MT, Pizzagalli DA (2019) The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry 85(6):443–453
Ruiz NAL, Del Ángel DS, Brizuela NO, Peraza AV, Olguín HJ, Soto MP, Guzmán DC (2022) Inflammatory process and immune system in major depressive disorder. Int J Neuropsychopharmacol 25(1):46–53
Bian H, Wang G, Huang J, Liang L, Zheng Y, Wei Y, Wang H, Xiao L, Wang H (2020) Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. J Neuroinflammation 17(1):166
Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S (2019) Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol 10:1511
Banagozar Mohammadi A, Torbati M, Farajdokht F, Sadigh-Eteghad S, Fazljou SMB, Vatandoust SM, Golzari SEJ, Mahmoudi J (2019) Sericin alleviates restraint stress induced depressive- and anxiety-like behaviors via modulation of oxidative stress, neuroinflammation and apoptosis in the prefrontal cortex and hippocampus. Brain Res 1715:47–56
Fang W, Goldberg ML, Pohl NM, Bi X, Tong C, Xiong B, Koh TJ, Diamond AM, Yang W (2010) Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 31(8):1360–1366
Vaváková M, Ďuračková Z, Trebatická J (2015) Markers of oxidative stress and neuroprogression in depression disorder. Oxid Med Cell Longev 2015:898393
Miyaguchi K (2004) Localization of selenium-binding protein at the tips of rapidly extending protrusions. Histochem Cell Biol 121:371–376
Cao P, Chen C, Liu A, Shan Q, Zhu X, Jia C, Peng X, Zhang M, Farzinpour Z, Zhou W, Wang H, Zhou JN, Song X, Wang L, Tao W, Zheng C, Zhang Y, Ding YQ, Jin Y, Xu L, Zhang Z (2021) Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 109(16):2573-2589.e9
Li S, Fang Y, Zhang Y, Song M, Zhang X, Ding X, Yao H, Chen M, Sun Y, Ding J, Wang Q, Lu M, Wu G, Hu G (2022) Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep 41(4):111532
Liu J, Mo JW, Wang X, An Z, Zhang S, Zhang CY, Yi P, Leong ATL, Ren J, Chen LY, Mo R, Xie Y, Feng Q, Chen W, Gao TM, Wu EX, Feng Y, Cao X (2022) Astrocyte dysfunction drives abnormal resting-state functional connectivity in depression. Sci Adv 8(46):eabo2098
Diaz-Castro B, Bernstein AM, Coppola G, Sofroniew MV, Khakh BS (2021) Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep 36(6):109508
Acknowledgements
The authors would like to thank the Research Center for Basic Sciences of Medicine of Guizhou Medical University for providing large-scale instrument and equipment.
Funding
This work was supported by the National Natural Science Foundation of China [22167008, 21867007, 21561006 and 12132006]; Key Laboratory of Infectious Immune and Antibody Engineering of Guizhou Province [KY [2020]012]; Guizhou Provincial Natural Science Foundation [[2019]1258 and ZK[2023]295]; Opening fund of Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica [BCMM202002]; Science and Technology Fund of Guizhou Provincial Health Commission [gzwkj2022-514]; Innovation and Entrepreneurship Training program for College students [202110660023]; and the Guizhou Provincial Natural Science Foundation for High-Level Innovative Talents and Teams [2016–5676, 2015–4021].
Author information
Authors and Affiliations
Contributions
Yi Jia, Xin Zhang and Yulian Chen: Conceptualization; Yi Jia, Xin Zhang, Yongmei Wang, Yang Liu, Jie Dai, Liangliang Zhang, Xian Wu, Jie Zhang, Hongxi Xiang and Yanping Yang: Methodology; Yi Jia, Jie Dai and Yulian Chen: Validation; Yi Jia, Xin Zhang, Yongmei Wang, Yang Liu Jie Dai, Liangliang Zhang, Xian Wu, Jie Zhang, Hongxi Xiang and Yanping Yang: Data curation; Yi Jia and Xin Zhang: Writing—original draft preparation; Yi Jia, Zhu Zeng and Yulian Chen: Writing—review and editing; Yi Jia, Xin Zhang, Yongmei Wang, Yang Liu and Jie Dai: Visualization; Yi Jia, Jie Dai and Yulian Chen: Supervision; Yi Jia, Jie Dai and Zhu Zeng: Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Guizhou Medical University (protocol code: 2021(300), date of approval: 25 March 2021). And the animal study protocol was approved by the Institutional Animal Care and Use Committee at Guizhou Medical University (protocol code 2,100,357, date of approval: 2 March 2021).
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, Y., Zhang, X., Wang, Y. et al. Knocking out Selenium Binding Protein 1 Induces Depressive-Like Behavior in Mice. Biol Trace Elem Res 202, 3149–3162 (2024). https://doi.org/10.1007/s12011-023-03894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03894-8