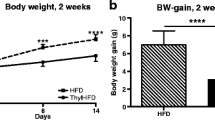
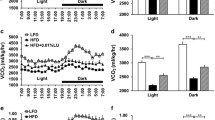
Abstract
Brown adipose tissue (BAT) and white adipose tissue (WAT) are known to regulate lipid metabolism. A lower amount of BAT compared to WAT, along with adipose tissue dysfunction, can result in obesity. Studies have shown that selenium supplementation protects against adipocyte dysfunction, decreases WAT triglycerides, and increases BAT triiodothyronine (T3). In this review, we discuss the relationship between selenium and lipid metabolism regulation through selenoprotein deiodinases and the role of deiodinases and thyroid hormones in the induction of adipose tissue thermogenesis. Upon 22 studies included in our review, we found that studies investigating the relationship between selenium and deiodinases demonstrated that selenium supplementation affects the iodothyronine deiodinase 2 (DIO2) protein and the expression of its associated gene, DIO2, proportionally. However, its effect on DIO1 is inconsistent while its effect on DIO3 activity is not detected. Studies have shown that the activity of deiodinases especially DIO2 protein and DIO2 gene expression is increased along with other browning markers upon white adipose tissue browning induction. Studies showed that thermogenesis is stimulated by the thyroid hormone T3 as its activity is correlated to the expression of other thermogenesis markers. A proposed mechanism of thermogenesis induction in selenium supplementation is by autophagy control. However, more studies are needed to establish the role of T3 and autophagy in adipose tissue thermogenesis, especially, since some studies have shown that thermogenesis can function even when T3 activity is lacking and studies related to autophagy in adipose tissue thermogenesis have contradictory results.




Similar content being viewed by others
References
World Health Organization (2021) World Health Statistics 2021: monitoring health for the SDGs, sustainable development goals. World Health Organization, Geneva
Miettinen S, Sarkanen JR, Ashammakhi N (2008) Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. In: Ashammakhi N, Reis R, Chiellini F (eds) Topics in tissue engineering. Oulu University, pp 1–26
Leitner BP, Huang S, Brychta RJ et al (2017) Mapping of human brown adipose tissue in lean and obese young men. PNAS 114:8649–8654. https://doi.org/10.1073/pnas.1705287114
Kotzbeck P, Giordano A, Mondini E et al (2018) Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 59:784–794. https://doi.org/10.1194/jlr.M079665
Dadson P, Jarna MPH, Din MU et al (2018) Brown adipose tissue lipid metabolism in morbid obesity: effect of bariatric surgery-induced weight loss. Diabetes Obes Metab 20:1280–1288. https://doi.org/10.1111/dom.13233
Softic S, Meyer JG, Wang G et al (2021) Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab 30:735–753. https://doi.org/10.1016/j.cmet.2019.09.003
Chondronikola M, Volpi E, Børsheim E et al (2016) Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23:1200–1206. https://doi.org/10.1016/j.cmet.2016.04.029
Wang Q, Zhang M, Xu M et al (2015) Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One 10:e0123795. https://doi.org/10.1371/journal.pone.0123795
Zimmermann R, Strauss JG, Haemmerle G et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science (80- ) 306:1383–1386. https://doi.org/10.1126/science.1100747
Briot A, Decaunes P, Volat F et al (2018) Senescence alters PPARγ (peroxisome proliferator–activated receptor gamma ) -dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arter Thromb Vasc Biol 38:1134–1146. https://doi.org/10.1161/ATVBAHA.118.310797
Bargut TCL, Souza-Mello V, Aguila MB et al (2017) Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig 31:20160051. https://doi.org/10.1515/hmbci-2016-0051
Wu J, Boström P, Sparks LM et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. https://doi.org/10.1016/j.cell.2012.05.016
Frontini A, Vitali A, Perugini J et al (2013) White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta Mol Cell Biol Lipids 1831:950–959. https://doi.org/10.1016/j.bbalip.2013.02.005
Cypess AM, Weiner LS, Roberts-Toler C et al (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Richard JE, López-Ferreras L, Chanclón B et al (2017) CNS β3-adrenergic receptor activation regulates feeding behavior, white fat browning, and body weight. Am J Physiol Endocrinol Metab 313:E344–E358. https://doi.org/10.1152/ajpendo.00418.2016
Ohno H, Shinoda K, Spiegelman BM et al (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15:395–404. https://doi.org/10.1016/j.cmet.2012.01.019
Zhang Y, Xie C, Wang H et al (2016) Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab 311:E530–E541. https://doi.org/10.1152/ajpendo.00094.2016
Fisher FF, Kleiner S, Douris N et al (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281. https://doi.org/10.1101/gad.177857.111
Boon MR, van den Berg SAA, Wang Y et al (2013) BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One 8:e74083. https://doi.org/10.1371/journal.pone.0074083
Cheng L, Wang J, Dai H et al (2021) Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 10:48–65. https://doi.org/10.1080/21623945.2020.1870060
Błazewicz A, Klatka M, Astel A et al (2015) Serum and urinary selenium levels in obese children: a cross-sectional study. J Trace Elem Med Biol 29:116–122. https://doi.org/10.1016/j.jtemb.2014.07.016
Rocourt CRB, Cheng W (2013) Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 5:1349–1365. https://doi.org/10.3390/nu5041349
Institute of Medicine (US) Panel on dietary antioxidants and related compounds (2000) selenium. In: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids 7:286–316. https://www.ncbi.nlm.nih.gov/books/NBK225483/pdf/Bookshelf_NBK225483.pdf
Delezie E, Rovers M, Van Der Aa A et al (2014) Comparing responses to different selenium sources and dosages in laying hens. Poult Sci 93:3083–3090. https://doi.org/10.3382/ps.2014-04301
Kohler LN, Foote J, Kelley CP et al (2018) Selenium and type 2 diabetes: systematic review. Nutrients 10:1924. https://doi.org/10.3390/nu10121924
Klein EA, Goodman PJ, Lucia MS et al (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301:39–51
Zhao Z, Barcus M, Kim J et al (2016) High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr 146:1625–1633. https://doi.org/10.3945/jn.116.229955
Guo L, Xiao J, Liu H et al (2020) Selenium nanoparticles alleviate hyperlipidemia and vascular injury in ApoE-deficient mice by regulating cholesterol metabolism and reducing oxidative stress. Metallomics 12:204–217. https://doi.org/10.1039/c9mt00215d
Papp LV, Lu J, Holmgren A et al (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806. https://doi.org/10.1089/ars.2007.1528
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Bevis LEM (2015) Soil-to-human mineral transmission with an emphasis on zinc, selenium, and iodine. Springer Sci Rev 3:77–96. https://doi.org/10.1007/s40362-014-0026-y
Liu J, Zheng F, Cheng R et al (2018) Site-specific incorporation of selenocysteine using an expanded genetic code and palladium-mediated chemical deprotection. J Am CHhem Soc 140:8807–8816. https://doi.org/10.1021/jacs.8b04603
Chung CZ, Krahn N (2022) The selenocysteine toolbox: a guide to studying the 21st amino acid. Arch Biochem Biophys 730:109421. https://doi.org/10.1016/j.abb.2022.109421
Hatfield DL, Schweizer U, Tsuji PA et al (2016) Selenium: its molecular biology and role in human health, 4th edn. Springer, New York
Gladyshev VN, Arnér ES, Berry MJ et al (2016) Selenoprotein gene nomenclature. J Biol Chem 291:24036–24040. https://doi.org/10.1074/jbc.M116.756155
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science (80- ) 300:1439–1443. https://doi.org/10.1126/science.1083516
Abou-rjeileh U, Contreras GA (2021) Redox regulation of lipid mobilization in adipose tissues. Antioxidants 10:1090
Mullur R, Liu Y, Brent GA (2014) Thyroid hormone action of metabolism. Physiol Rev 94:355–382. https://doi.org/10.1152/physrev.00030.2013
Tinkov AA, Ajsuvakova OP, Filippini T et al (2020) Selenium and selenoproteins in adipose tissue physiology and obesity. Biomolecules 10:658
Zhao H, Li K, Tang J et al (2015) Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr 145:1394–1401. https://doi.org/10.3945/jn.115.211318
Sabatino L, Vassalle C, Del Seppia C et al (2021) Deiodinases and the three types of thyroid hormone deiodination reactions. Endocrinol Metab 36:952–964. https://doi.org/10.3803/EnM.2021.1198
Murakami M, Kamiya Y, Morimura T et al (2001) Thyrotropin receptors in brown adipose tissue: thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinol 142:1195–1201. https://doi.org/10.1210/endo.142.3.8012
de Jesus LA, Carvalho SD, Ribeiro MO et al (2001) The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Investig 108:1379–1385. https://doi.org/10.1172/jci13803
Dittner C, Lindsund E, Cannon B et al (2019) At thermoneutrality, acute thyroxine-induced thermogenesis and pyrexia are independent of. Mol Metab 25:20–34. https://doi.org/10.1016/j.molmet.2019.05.005
Broeders EPM, Vijgen GHEJ, Havekes B et al (2016) Thyroid hormone activates brown adipose tissue and increases non-shivering thermogenesis - a cohort study in a group of thyroid carcinoma patients. PLoS One 11:e0145049. https://doi.org/10.1371/journal.pone.0145049
Skarulis MC, Celi FS, Mueller E et al (2010) Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab 95:256–262. https://doi.org/10.1210/jc.2009-0543
Harris SE, De Blasio MJ, Zhao X et al (2020) Thyroid deficiency before birth alters the adipose transcriptome to promote overgrowth of white adipose tissue and impair thermogenic capacity. Thyroid 30:794–805. https://doi.org/10.1089/thy.2019.0749
Araki O, Ying H, Zhu XG et al (2009) Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol Endocrinol 23:308–315. https://doi.org/10.1210/me.2008-0311
Bernet VJ (2019) Thyroid hormone misuse and abuse. Endocrine 66:79–86. https://doi.org/10.1007/s12020-019-02045-1
Nido SA, Shituleni SA, Mengistu BM et al (2016) Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res 171:399–409. https://doi.org/10.1007/s12011-015-0552-8
Hauffe R, Rath M, Agyapong W et al (2022) Obesity hinders the protective effect of selenite supplementation on insulin signaling. Antioxidants 11:862
Laureano-Melo R, Império GE, Kluck GEG et al (2020) Selenium supplementation during pregnancy and lactation promotes metabolic changes in Wistar rats’ offspring. Clin Exp Pharmacol 47:1272–1282. https://doi.org/10.1111/1440-1681.13268
Chadio SE, Kotsampasi BM, Menegatos JG et al (2006) Effect of selenium supplementation on thyroid hormone levels and selenoenzyme activities in growing lambs. Biol Trace Elem Res 109:145–154. https://doi.org/10.1385/BTER:109:2:145
Page M, Moher D, Bossuyt P et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J 372:n160. https://doi.org/10.1136/bmj.n.160.PRISMA
Duntas LH, Brenta G (2018) A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol 9:511. https://doi.org/10.3389/fendo.2018.00511
Ducharme NA, Bickel PE (2008) Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinology 149:942–949. https://doi.org/10.1210/en.2007-1713
Saponaro C, Gaggini M, Carli F et al (2015) The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 9453–9474. https://doi.org/10.3390/nu7115475
Gambo Y, Matsumura M, Fujimori K (2016) Triiodothyronine enhances accumulation of intracellular lipids in adipocytes through thyroid hormone receptor a via direct and indirect mechanisms. Mol Cell Endocrinol 431:1–11. https://doi.org/10.1016/j.mce.2016.04.023
Xiong S, Chirala SS, Hsu MH et al (1998) Identification of thyroid hormone response elements in the human fatty acid synthase promoter. Proc Natl Acad Sci USA 95:12260–12265. https://doi.org/10.1073/pnas.95.21.12260
Weinhofer I, Kunze M, Rampler H et al (2008) Distinct modulatory roles for thyroid hormone receptors TR a and TR b in SREBP1-activated ABCD2 expression. Eur J Cell Biol 87:933–945. https://doi.org/10.1016/j.ejcb.2008.08.002
Zhang Y, Yin L, Hillgartner FB (2003) SREBP-1 integrates the actions of thyroid hormone, insulin, cAMP, and medium-chain fatty acids on ACCα transcription in hepatocytes. J Lipid Res 44:356–368. https://doi.org/10.1194/jlr.M200283-JLR200
Yau WW, Singh BK, Lesmana R et al (2019) Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 15:131–150. https://doi.org/10.1080/15548627.2018.1511263
Klieverik LP, Coomans CP, Endert E et al (2009) Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology 150:5639–5648. https://doi.org/10.1210/en.2009-0297
Schweiger M, Eichmann TO, Taschler U et al (2014) Measurement of lipolysis. Methods Enzym 538:171–193. https://doi.org/10.1016/B978-0-12-800280-3.00010-4
Grasselli E, Voci A, Demori I et al (2016) Triglyceride mobilization from lipid droplets sustains the anti-steatotic action of iodothyronines in cultured rat hepatocytes. Front Physiol 6:416. https://doi.org/10.3389/fphys.2015.00418
Iannucci LF, Cioffi F, Senese R et al (2023) Metabolomic analysis shows differential hepatic effects of T 2 and T 3 in rats after short-term feeding with high fat diet. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-02205-1
Chadio SE, Pappas AC, Papanastasatos A et al (2015) Effects of high selenium and fat supplementation on growth performance and thyroid hormones concentration of broilers. J Trace Elem Med Biol 29:202–207. https://doi.org/10.1016/j.jtemb.2014.09.010
Combs GFJ, Midthune DN, Patterson KY et al (2009) Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr 89:1808–1814. https://doi.org/10.3945/ajcn.2008.27356
Cicatiello AG, Di GD, Dentice M (2018) Metabolic effects of the intracellular regulation of thyroid hormone: old players, new concepts. Front Endocrinol 9:474. https://doi.org/10.3389/fendo.2018.00474
Sinha R, Yen PM (2018) Cellular action of thyroid hormone. 1:1–55. https://www.ncbi.nlm.nih.gov/books/NBK285568
Weitzel JM, Iwen KAH, Seitz HJ (2003) Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol 88:121–128
Darras VM, Houbrechts AM, Van HSLJ (2015) Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor-mediated impact on vertebrate development. Biochim Biophys Acta - Gene Regul Mech 1849:130–141. https://doi.org/10.1016/j.bbagrm.2014.05.004
Ribeiro MO, Bianco SDC, Kaneshige M et al (2010) Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-β isoform specific and required for adaptive thermogenesis. Endocrinology 151:432–440. https://doi.org/10.1210/en.2009-0667
Ribeiro MO, Carvalho SD, Schultz JJ et al (2001) Thyroid hormone – sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform – specific. J Clin Investig 108:97–105. https://doi.org/10.1172/JCI200112584
Kasher-Meron M, Youn DY, Zong H et al (2019) Lipolysis defect in white adipose tissue and rapid weight regain. Am J Physiol Endocrinol Metab 317:E185–E193
Cheng Y, Meng Q, Wang C et al (2010) Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 ( UCP1) in brown adipose tissue. Diabetes 59:17–25. https://doi.org/10.2337/db09-0929.Y.C
Nannipieri M, Cecchetti F, Anselmino M et al (2009) Expression of thyrotropin and thyroid hormone receptors in adipose tissue of patients with morbid obesity and/or type 2 diabetes: effects of weight loss. IJO 33:1001–1006. https://doi.org/10.1038/ijo.2009.140
Liu Y, Schultz JJ, Brent GA (2003) A thyroid hormone receptor α gene mutation ( P398H ) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem 278:38913–38920. https://doi.org/10.1074/jbc.M306120200
Geillinger KE, Rathmann D, Josef K et al (2014) Hepatic metabolite profiles in mice with a suboptimal selenium status. J Nutr Biochem 25:914–922. https://doi.org/10.1016/j.jnutbio.2014.04.003
Calvo RM, Obregon MJ (2011) Presence and regulation of D1 and D2 deiodinases in rat white adipose tissue. Metabolism 60:1207–1210. https://doi.org/10.31857/s013116462104007x
Hernandez A, Martinez ME, Fiering S et al (2006) Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484. https://doi.org/10.1172/JCI26240.476
Wu Z, Martinez ME, Germain DLS et al (2017) Type 3 deiodinase role on central thyroid hormone action affects the leptin-melanocortin system and circadian activity. Endocrinology 158:419–430. https://doi.org/10.1210/en.2016-1680
Jílková ZM, Pavelka S, Flachs P et al (2010) Modulation of type I iodothyronine 5’-deiodinase activity in white adipose tissue by nutrition: possible involvement of leptin. Physiol Res 59:561–569. https://doi.org/10.33549/physiolres.931866
Picó C, Palou M, Amadora C et al (2022) Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord 13–30. https://doi.org/10.1007/s11154-021-09687-5
Dobrzyn P, Dobrzyn A, Miyazaki M et al (2004) Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. PNAS 101:6409–6414. https://doi.org/10.1073/pnas.0401627101
Kim E, Lee J, Ntambi JM et al (2011) Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits bene fi cial lipid metabolic effects in vitro. Eur J Pharmacol 672:38–44. https://doi.org/10.1016/j.ejphar.2011.09.172
Bruinstroop E, Zhou J, Tripathi M et al (2021) Early induction of hepatic deiodinase type 1 inhibits hepatosteatosis during NAFLD progression. Mol Metab 53:2–8. https://doi.org/10.1016/j.molmet.2021.101266
Charalambous M, Ferron SR, Rocha ST et al (2012) Imprinted gene dosage is critical for the transition to independent life. Cell Metab 15:209–221. https://doi.org/10.1016/j.cmet.2012.01.006
Johann K, Cremer AL, Fischer AW et al (2019) Thyroid-hormone-induced browning of white adipose tissue does not contribute to thermogenesis and glucose consumption. Cell Rep 27:3385–3400. https://doi.org/10.1016/j.celrep.2019.05.054
Akahoshi N, Anan Y, Hashimoto Y et al (2019) Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J Nutr Biochem 69:120–129. https://doi.org/10.1016/j.jnutbio.2019.03.020
Sun L, Goh HJ, Govindharajulu P et al (2020) A feedforward loop within the thyroid-brown fat axis facilitates thermoregulation. Sci Rep 10:9661. https://doi.org/10.1038/s41598-020-66697-0
Cioffi F, Gentile A, Silvestri E et al (2018) Effect of iodothyronines on thermogenesis: focus on brown adipose tissue. Front Endocrinol 9:254. https://doi.org/10.3389/fendo.2018.00254
Weiner J, Kranz M, Klöting N et al (2016) Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci Rep 6:38124. https://doi.org/10.1038/srep38124
Shigematsu M, Yamada T, Wong YY et al (2016) Dietary regulation of Ucp2 and Ucp3 expressions in white adipose tissues of beef cattle. Can J Anim Sci 96:457–460. https://doi.org/10.1139/cjas-2016-0020
Medina-Gomez G, Calvo RM, Obregon MJ (2008) Thermogenic effect of triiodothyroacetic acid at low doses in rat adipose tissue without adverse side effects in the thyroid axis. Am J Physiol Endocrinol Metab 294:688–697. https://doi.org/10.1152/ajpendo.00417.2007
Wulf A, Harneit A, Kröger M et al (2008) T3-mediated expression of PGC-1α via a far upstream located thyroid hormone response element. Mol Cell Endocrinol 287:90–95. https://doi.org/10.1016/j.mce.2008.01.017
Akarsu E, Korkmaz H, Oguzkan Balci S et al (2015) Subcutaneous adipose tissue type II deiodinase gene expression reduced in obese individuals with metabolic syndrome. Exp Clin Endocrinol Diabetes 124:11–15. https://doi.org/10.1055/s-0035-1564129
Christoffolete MA, Linardi CCG, De Jesus L et al (2004) Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53:577–584. https://doi.org/10.2337/diabetes.53.3.577
Martinez-demena R, Anedda A, Cadenas S et al (2015) Molecular and cellular endocrinology TSH effects on thermogenesis in rat brown adipocytes. Mol Cell Endocrinol 404:151–158. https://doi.org/10.1016/j.mce.2015.01.028
Chrysovergis K, Wang X, Kosak J et al (2014) NAG-1 / GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes 38:1555–1564. https://doi.org/10.1038/ijo.2014.27
Hoeke G, Kooijman S, Boon MR et al (2016) Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res 118:173–182. https://doi.org/10.1161/CIRCRESAHA.115.306647
Calvo RM, Garcia L, Vesperinas G et al (2017) Deiodinases in human adipose tissue from obese patients. Differences by gender and anatomical depot. JSM Thyroid Disord Manag 2:1009
De VEM, Van BHC, Van WACWA et al (2020) Regulation of type 3 deiodinase in rodent liver and adipose tissue during fasting. Endocr Connect 9:552–562. https://doi.org/10.1530/EC-20-0189
Chen Y, Yang Q, Hu Y et al (2021) Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance. Nat Commun 12:6845. https://doi.org/10.1038/s41467-021-27171-1
Lénárt K, Bankó C, Ujlaki G et al (2022) Tissue transglutaminase knock-out preadipocytes and beige cells of epididymal fat origin possess decreased mitochondrial functions required for thermogenesis. Int J Mol Sci. https://doi.org/10.3390/ijms23095175
Kӧhrle J, Frӓdrich C (2022) Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic Biol Med 193:59–79. https://doi.org/10.1016/j.freeradbiomed.2022.09.024
Sunde RA, Raines AM (2011) Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv Nutr 2:138–150. https://doi.org/10.3945/an.110.000240.138
Fontenelle LC, Feitosa MM, Freitas TEC et al (2021) Selenium status and its relationship with thyroid hormones in obese women. Clin Nutr ESPEN 41:398–404. https://doi.org/10.1016/j.clnesp.2020.10.012
Vadhanavikit S, Ganther HE (1993) Selenium requirements of rats for normal hepatic and thyroidal 5’-deiodinase (type I) activities. J Nutr 123:1124–1128. https://doi.org/10.1093/jn/123.6.1124
Bates JM, Spate VL, Morris JS et al (2000) Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology 141:2490–2500. https://doi.org/10.1210/endo.141.7.7571
Cao L, Zhang L, Zeng H et al (2017) Analyses of selenotranscriptomes and selenium concentrations in response to dietary selenium deficiency and age reveal common and distinct patterns by tissue and sex in telomere-dysfunctional mice. J Nutr 147:1858–1866. https://doi.org/10.3945/jn.117.247775
Liang Y, Lin SL, Wang CW et al (2014) Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res 160:41–48. https://doi.org/10.1007/s12011-014-0024-6
Abo EL-Magd NF, D EP, Barbosa PO et al (2022) Selenium, as selenite, prevents adipogenesis by modulating selenoproteins gene expression and oxidative stress-related genes. Nutrition 93:111424https://doi.org/10.1016/j.nut.2021.111424
Steinbrenner H, Micoogullari M, Hoang NA et al (2019) Redox biology selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol 20:489–495. https://doi.org/10.1016/j.redox.2018.11.004
Wang Y, Gao X, Pedram P et al (2016) Significant beneficial association of high dietary selenium intake with reduced body fat in the CODING study. Nutrients 8:24. https://doi.org/10.3390/nu8010024
Huang SA (2005) Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15:875–881
Lucroy MD (2008) Tumor markers. In: Clinical biochemistry of domestic animals, 6th Ed. Elsevier Inc, pp 751–767
Dentice M, Salvatore D (2011) Deiodinases: the balance of thyroid hormone Local impact of thyroid hormone inactivation. J Endocrinol 209:273–282. https://doi.org/10.1530/JOE-11-0002
Krause K (2019) Novel aspects of white adipose tissue browning by thyroid hormones author how do thyroid hormones induce adipose tissue browning ? Exp Clin Endocrinol Diabetes 128:446–449. https://doi.org/10.1055/a-1020-5354
Lindsey RC, Mohan S (2022) Thyroid hormone acting via TRβ induces expression of browning genes in mouse bone marrow adipose tissue. Endocrine 56:109–120. https://doi.org/10.1007/s12020-017-1265-x
Lisboa PC, Conceição EPS, de Oliveira E et al (2015) Postnatal overnutrition programs the thyroid hormone metabolism and function in adulthood. J Endocrinol 226:219–226. https://doi.org/10.1530/JOE-15-0237
Golozoubova V, Gullberg H, Matthias A et al (2004) Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. J Mol Endocrinol 18:384–401. https://doi.org/10.1210/me.2003-0267
Billon C, Canaple L, Fleury S et al (2014) TRα protects against atherosclerosis in male mice: for TRα in mice. Endocrinology 155:2735–2745. https://doi.org/10.1210/en.2014-1098
Gullberg H, Rudling M, Salto C et al (2002) Requirement for thyroid hormone receptor β in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol 16:1767–1777. https://doi.org/10.1210/me.2002-0009
Nascimento EBM, Sparks LM, Divoux A et al (2018) Genetic markers of brown adipose tissue identity and in vitro brown adipose tissue activity in humans. Obesity 26:135–140. https://doi.org/10.1002/oby.22062
Tabuchi C, Sul HS (2021) Signaling pathways regulating thermogenesis. Front Endocrinol 12:595020. https://doi.org/10.3389/fendo.2021.595020
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. https://doi.org/10.1152/physrev.00015.2003
Pagnon J, Matzaris M, Stark R et al (2012) Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 153:4278–4289. https://doi.org/10.1210/en.2012-1127
Barneda D, Frontini A, Cinti S et al (2013) Dynamic changes in lipid droplet-associated proteins in the “browning” of white adipose tissues. Biochim Biophys Acta Mol Cell Biol Lipids 1831:924–933. https://doi.org/10.1016/j.bbalip.2013.01.015
Merlin J, Sato M, Chia LY et al (2018) Rosiglitazone and a β3-adrenoceptor agonist are both required for functional browning of white adipocytes in culture. Front Endocrinol 9:249. https://doi.org/10.3389/fendo.2018.00249
Hondares E, Rosell M, Díaz-Delfín J et al (2011) Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem 286:43112–43122. https://doi.org/10.1074/jbc.M111.252775
Zeng C, Chen M (2022) Progress in nonalcoholic fatty liver disease: SIRT family regulaTES MITOCHONDRIAL BIOGENesis. Biomolecules 12:1079. https://doi.org/10.3390/biom12081079
Echeverría F, Ortiz M, Valenzuela R et al (2016) Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: relationship to tissue development and aging. Prostaglandins Leukot Essent Fat Acids 114:28–34. https://doi.org/10.1016/j.plefa.2016.10.001
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370. https://doi.org/10.1016/j.cmet.2005.05.004
Liang H, Ward WF (2006) PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30:145–151. https://doi.org/10.1152/advan.00052.2006
Boström P, Wu J, Jedrychowski MP et al (2012) A PGC1α-dependent myokine that drives browning of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777.A
Wang L, Teng R, Di L et al (2013) PPARa and sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 62:4122–4131. https://doi.org/10.2337/db13-0518
Yan Y, Yang D, Wen P et al (2022) Expression analysis of irisin during different development stages of skeletal muscle in mice. Gene Expr Patterns 46:119287. https://doi.org/10.1016/j.gep.2022.119287
Luo X, Li J, Zhang H et al (2022) Irisin promotes the browning of white adipocytes tissue by AMPK α 1 signaling pathway. Res Vet Sci 152:270–276. https://doi.org/10.1016/j.rvsc.2022.08.025
Vliora M, Grillo E, Corsini M et al (2022) Irisin regulates thermogenesis and lipolysis in 3T3-L1 adipocytes. BBA - Gen Subj 1866:130085. https://doi.org/10.1016/j.bbagen.2022.130085
Wu L, Zhang L, Li B et al (2018) AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol 9:122. https://doi.org/10.3389/fphys.2018.00122
Neill HMO, Holloway GP, Steinberg GR (2013) AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol 366:135–151. https://doi.org/10.1016/j.mce.2012.06.019
Chen Y, Ding J, Zhao Y et al (2021) Irisin induces white adipose tissue browning in mice as assessed by magnetic resonance imaging. Exp Biol Med 246:1597–1606. https://doi.org/10.1177/15353702211006049
Jiang C, Cano-vega MA, Yue F et al (2017) Dibenzazepine-loaded nanoparticles induce local browning of white adipose tissue to counteract obesity. Mol Ther 25:1718–1729. https://doi.org/10.1016/j.ymthe.2017.05.020
Hedesan OC, Fenzl A, Digruber A et al (2018) Parathyroid hormone induces a browning program in human white adipocytes. Int J Obes 4–9. https://doi.org/10.1038/s41366-018-0266-z
Moreno-Navarrete JM, Serino M, Blasco-Baque V et al (2018) Gut microbiota interacts with markers of adipose tissue browning, insulin action and plasma acetate in morbid obesity. Mol Nutr Food Res 62:1700721. https://doi.org/10.1002/mnfr.201700721
Gavaldà-navarro A, Moreno-navarrete JM (2016) Lipopolysaccharide-binding protein is a negative regulator of adipose tissue browning in mice and humans. Diabetologia 59:2208–2218. https://doi.org/10.1007/s00125-016-4028-y
Li B, Zhang M, Duan Y et al (2020) Pyrazolone derivative C29 protects against HFD-induced obesity in mice via activation of AMPK in adipose tissue. Acta Pharmacol Sin 42:964–974. https://doi.org/10.1038/s41401-020-00524-0
Wu X, Li J, Chang K et al (2021) Molecular and cell biology of lipids histone H3 methyltransferase Ezh2 promotes white adipocytes but inhibits brown and beige adipocyte differentiation in mice. BBA - Mol Cell Biol Lipids 1866:158901. https://doi.org/10.1016/j.bbalip.2021.158901
Hanatani S, Motoshima H, Takaki Y et al (2016) Acetate alters expression of genes involved in beige adipogenesis in 3T3 L1 cells and obese KK Ay mice. J Clin Biochem Nutr 59:207–214. https://doi.org/10.3164/jcbn.16
Yan C, Zeng T, Lee K et al (2021) Peripheral-specific Y1 receptor antagonism increases thermogenesis and protects against diet-induced obesity. Nat Commun 12:2622. https://doi.org/10.1038/s41467-021-22925-3
Sandhu B, Perez-matos MC, Tran S et al (2021) Global deletion of NTPDase3 protects against diet-induced obesity by increasing basal energy metabolism. Metabolism 118:154731. https://doi.org/10.1016/j.metabol.2021.154731
Heeren J, Scheja L (2018) Brown adipose tissue and lipid metabolism. Curr Opin Lipidol 29:180–185. https://doi.org/10.1097/MOL.0000000000000504
De MRM, Scanlan TS, Obregon M (2010) The T3 Receptor β1 isoform regulates UCP1 and D2 deiodinase in rat brown adipocytes. Endocrinology 151:5074–5083. https://doi.org/10.1210/en.2010-0533
Fonseca TL, Werneck-de-castro JP, Castillo M et al (2014) Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes 63:1594–1604. https://doi.org/10.2337/db13-1768
De OMAP, Gutiérrez-mariscal M, Salmerón-jiménez MF et al (2019) Voluntary exercise-induced activation of thyroid axis and reduction of white fat depots is attenuated by chronic stress in a sex dimorphic pattern in adult rats. Front Endocrinol 10:418. https://doi.org/10.3389/fendo.2019.00418
Bradley D, Liu J, Blaszczak A et al (2018) Adipocyte DIO2 expression increases in human obesity but is not related to systemic insulin sensitivity. J Diabetes Res 2018:2564652. https://doi.org/10.1155/2018/2464652
Davis RAH, Deemer SE, Bergeron JM et al (2019) Dietary R, S -1, 3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. FASEB J 33:2409–2421. https://doi.org/10.1096/fj.201800821RR
Havekes B, Schrauwen P, Broeders EPM et al (2015) Clinical and translational report the bile acid chenodeoxycholic acid increases human brown adipose tissue activity clinical and translational report the bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22:418–426. https://doi.org/10.1016/j.cmet.2015.07.002
Yang X, Sui W, Zhang M et al (2017) Switching harmful visceral fat to beneficial energy combustion improves metabolic dysfunctions. JCI Insight 2:e89044. https://doi.org/10.1172/jci.insight.89044
Ayala-moreno R, Racotta R, Anguiano B et al (2013) Perinatal undernutrition programmes thyroid function in the adult rat offspring. Br J Nutr 110:2207–2215. https://doi.org/10.1017/S0007114513001736
Shu L, Hoo RLC, Wu X et al (2017) A-FABP mediates adaptive thermogenesis by promoting intracellular activation of thyroid hormones in brown adipocytes. Nat Commun 8:14147. https://doi.org/10.1038/ncomms14147
Seale LA, Ogawa-wong AN, Watanabe LM et al (2021) Adaptive thermogenesis in a mouse model lacking selenoprotein biosynthesis in brown adipocytes. Int J Mol Sci 22:611
Bank JHH, Kemmling J, Rijntjes E et al (2015) Thyroid hormone status affects expression of daily torpor and gene transcrip- tion in djungarian hamsters (Phodopus sungorus). Horm Behav 75:120–129. https://doi.org/10.1016/j.yhbeh.2015.09.006
Kovaničová Z, Kurdiová T, Baláž M et al (2020) Cold exposure distinctively modulates parathyroid and thyroid hormones in cold- acclimatized and non-acclimatized humans. Endocrinol 161:bqaa051. https://doi.org/10.1210/endocr/bqaa051
Palou M, Priego T, Romero M et al (2014) Moderate calorie restriction during gestation programs offspring for lower BAT thermogenic capacity driven by thyroid and sympathetic signaling. Int J Obes 39:339–345. https://doi.org/10.1038/ijo.2014.56
Fonseca TL, Werneck-de-castro JP, Castillo M et al (2014) Tissue-specific inactivation of type II deiodinase reveals multi-level control of fatty acid oxidation by thyroid hormone in the mouse division of endocrinology, Diabetes and Metabolism, University of Miami Miller School of Medicine, Miami, Florida Bi. Diabetes 63:1594–1604
Transl J, Chen H, Gan Q et al (2019) A novel role of glutathione S ‑ transferase A3 in inhibiting hepatic stellate cell activation and rat hepatic fibrosis. J Transl Med 17:28-. https://doi.org/10.1186/s12967-019-2027-8
Suzuki M, Murakami T, Cheng J et al (2015) ELMOD2 is anchored to lipid droplets by palmitoylation and regulates adipocyte triglyceride lipase recruitment. Mol Biol Cell 26:2333–2342. https://doi.org/10.1091/mbc.E14-11-1504
Jedrychowski MP, Lu GZ, Szpyt J et al (2020) Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. PNAS 117:10789–10796. https://doi.org/10.1073/pnas.2001387117
Reid ME, Stratton MS, Lillico AJ et al (2004) A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol 18:69–74. https://doi.org/10.1016/j.jtemb.2004.03.004
Ikeda K, Yamada T (2020) UCP1 Dependent and independent thermogenesis in brown and beige adipocytes. Front Endocrinol 11:498. https://doi.org/10.3389/fendo.2020.00498
Ikeda K, Kang Q, Yoneshiro T et al (2018) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23:1454–1465. https://doi.org/10.1038/nm.4429.UCP1-independent
Ribeiro MO, Lebrun FLAS, Christoffolete MA et al (2000) Evidence of UCP1-independent regulation of norepinephrine-induced thermogenesis in brown fat. Am J Physiol Endocrinol Metab 279:314–322
Lettieri-Barbato D (2019) Redox control of non-shivering thermogenesis. Mol Metab 25:11–19. https://doi.org/10.1016/j.molmet.2019.04.002
Endo T, Kobayashi T (2008) Thyroid-stimulating hormone receptor in brown adipose tissue is involved in the regulation of thermogenesis. Am J Physiol Endocrinol Metab 295:514–518. https://doi.org/10.1152/ajpendo.90433.2008
Kim MS, Hu HH, Aggabao PC et al (2014) Presence of brown adipose tissue in an adolescent. J Clin Endocrinol Metab 99:1686–1690. https://doi.org/10.1210/jc.2014-1343
Lundbäck V, Marcus C (2020) Adipose-specific inactivation of thyroid stimulating hormone receptors in mice modifies body weight, temperature and gene expression in adipocytes. Physiol Rep e14538. https://doi.org/10.14814/phy2.14538
Zhang J, Wu H, Ma S et al (2020) TSH promotes adiposity by inhibiting the browning of white fat. Adipocyte 9:264–278. https://doi.org/10.1080/21623945.2020.1783101
Ferhat M, Funai K, Boudina S (2019) Autophagy in adipose tissue physiology and pathophysiology. Antioxid Redox Signal 31:487–501. https://doi.org/10.1089/ars.2018.7626
Nguyen MT, Da LD, Nguyen NT et al (2021) Thyroid hormone induces Ca 2 + -mediated mitochondrial activation in brown adipocytes. Int J Mol Sci 22:8640
Xu Q, Mariman ECM, Roumans NJT et al (2018) Adipose tissue autophagy related gene expression is associated with glucometabolic status in human obesity. Adipocyte 7:12–19. https://doi.org/10.1080/21623945.2017.1394537
Sakane S, Hikita H, Shirai K et al (2021) White adipose tissue autophagy and adipose-liver crosstalk exacerbate nonalcoholic fatty liver disease in mice. Cell Mol Gastroenterol Hepatol 12:1683–1699. https://doi.org/10.1016/j.jcmgh.2021.07.008
Mattar P, Yuri G, Briones L et al (2020) Calcium-sensing receptor in adipose tissue: possible association with obesity-related elevated autophagy. Int J Mol Sci 21:7617
Mattar P, Bravo-sagua R, Tobar N et al (2018) Autophagy mediates calcium-sensing receptor-induced TNF α production in human preadipocytes. BBA - Mol Basis Dis 1864:3585–3594. https://doi.org/10.1016/j.bbadis.2018.08.020
Altshuler-keylin S, Shinoda K, Hasegawa Y et al (2016) Beige adipocyte maintenance is regulated by autophagy- induced mitochondrial clearance. Cell Metab 24:402–419. https://doi.org/10.1016/j.cmet.2016.08.002.Beige
Lu X, Altshuler-Keylin S, Wang Q et al (2018) Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci Signal 11:eaap8526. https://doi.org/10.1126/scisignal.aap8526.Mitophagy
Kim D, Kim J, Kang Y et al (2019) Suppression of brown adipocyte autophagy improves energy metabolism by regulating mitochondrial turnover. Int J Mol Sci 20:3520. https://doi.org/10.3390/ijms20143520
Ni H, Xu S, Chen H et al (2020) Synthesis and secretion through regulating the autophagy-lysosomal machinery. Arter Thromb Vasc Biol is 40:2054–2069. https://doi.org/10.1161/ATVBAHA.120.314053
Ziqubu K, Dludla P V, Mthembu SXH et al (2023) An insight into brown / beige adipose tissue whitening, a metabolic complication of obesity with the multifactorial origin. Front Endocrinol 1114767. https://doi.org/10.3389/fendo.2023.1114767
Yau WW, Bay H, Singh K (2021) Chronic cold exposure induces autophagy to promote fatty acid oxidation, mitochondrial turnover, and thermogenesis in brown adipose tissue. iScience 24:102434. https://doi.org/10.1016/j.isci.2021.102434
Funding
The funding of this study was supported by the world-class research 2022 Ministry of Education, Culture, Research, and Technology to Ronny Lesmana.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception and design. Material preparation and article collection were performed by Yasmin Anissa R. Ruswandi. The first draft of the manuscript was written by Yasmin Anissa R. Ruswandi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruswandi, Y.A.R., Lesmana, R., Rosdianto, A.M. et al. Understanding the Roles of Selenium on Thyroid Hormone-Induced Thermogenesis in Adipose Tissue. Biol Trace Elem Res 202, 2419–2441 (2024). https://doi.org/10.1007/s12011-023-03854-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03854-2