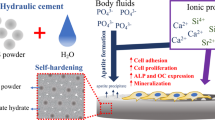
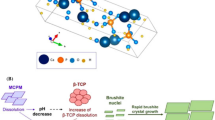
Abstract
New bone cement type that combines Sr2 + /Mg2 + or Sr2 + /Zn2 + co-substituted nano-hydroxyapatite (n-HAs) with calcium phosphate dibasic and chitosan/gelatin polymers was developed to increase adhesion and cellular response. The cements were physicochemically described and tested in vitro using cell cultures. All cements exhibited quite hydrophilic and had high washout resistance. Cement releases Ca2 + , Mg2 + , Sr2 + , and Zn2 + in concentrations that are suitable for osteoblast proliferation and development. All of the cements stimulated cell proliferation in fibroblasts, endothelial cells, and osteoblasts, were non-cytotoxic, and produced apatite. Cements containing co-substituted n-HAs had excellent cytocompatibility, which improved osteoblast adhesion and cell proliferation. These cements had osteoinductive potential, stimulating extracellular matrix (ECM) mineralization and differentiation of MC3T3-E1 cells by increasing ALP and NO production. The ions Ca2 + , Mg2 + , Zn2 + , and Sr2 + appear to cooperate in promoting osteoblast function. The C3 cement (HA-SrMg5%), which was made up of n-HA co-substituted with 5 mol% Sr and 5 mol% Mg, showed exceptional osteoinductive capacity in terms of bone regeneration, indicating that this new bone cement could be a promising material for bone replacement.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available at Alexa Magalhaes Dias’s repository, https://repositorio.ufmg.br/handle/1843/35912.
Materials Availability
The biological materials used were supplied by Rio de Janeiro Cell Bank (Brazil).
References
Cacciotti I (2016) Cationic and anionic substitutions in hydroxyapatite, in: Antoniac I (Ed.) Handbook of bioceramics and biocomposites. Springer Cham Switzerland. pp 145–211. https://doi.org/10.1007/978-3-319-12460-5_7
Azizeh-Mitra Y, Hassane O, Rosa A et al (2014) Physical and biological characteristics of nanohydroxyapatite and bioactive glasses used for bone tissue engineering. Nanotechnol Rev 3:527–552. https://doi.org/10.1515/ntrev-2014-0013
Chadha RK, Singh KL, Sharma C et al (2020) Effect of microwave and conventional processing techniques on mechanical properties of strontium substituted hydroxyapatite. Ceram Int 46:1091–1098. https://doi.org/10.1016/j.ceramint.2019.09.076
Haider A, Haider S, Han SS, Kang I-K (2017) Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: a review. RSC Adv 7:7442–7458. https://doi.org/10.1039/C6RA26124H
Begam H, Kundu B, Chanda A, Nandi SK (2017) MG63 osteoblast cell response on Zn doped hydroxyapatite (HAp) with various surface features. Ceram Int 43:3752–3760. https://doi.org/10.1016/j.ceramint.2016.12.010
Cox SC, Jamshidi P, Grover LM, Mallick KK (2014) Preparation and characterisation of nanophase Sr Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater Sci Eng C Mater Biol Appl 35:106–114. https://doi.org/10.1016/j.msec.2013.10.015
Frasnelli M, Cristofaro F, Sglavo VM, Dirè S et al (2017) Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater Sci Eng C Mater Biol Appl 1(71):653–662. https://doi.org/10.1016/j.msec.2016.10.047
Garbo C, Locs J, D’Este M, Demazeau G, Mocanu A et al (2020) Advanced Mg, Zn, Sr, Si multi-substituted hydroxyapatites for bone regeneration. Int J Nanomedicine 13(15):1037–1058. https://doi.org/10.2147/IJN.S226630
Šupová M (2015) Substituted hydroxyapatites for biomedical applications: a review. Ceram Int 41:9203–9231. https://doi.org/10.1016/j.ceramint.2015.03.316
Boanini E, Gazzano M, Bigi A (2010) Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater 6:1882–1894. https://doi.org/10.1016/j.actbio.2009.12.041
Okada M, Matsumoto T (2015) Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn Dent Sci Rev 51:85–95. https://doi.org/10.1016/j.jdsr.2015.03.004
Marx D, Rahimnejad YA, Papini MT (2020) A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep 12:100273. https://doi.org/10.1016/j.bonr.2020.100273
Yamaguchi M, Weitzmann MN (2011) Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol Cell Biochem 355:179–86. https://doi.org/10.1007/s11010-011-0852-z
Li Y, Yue J, Liu Y, Wu J, Guan M et al (2021) Strontium regulates stem cell fate during osteogenic differentiation through asymmetric cell division. Acta Biomater 119:432–443. https://doi.org/10.1016/j.actbio.2020.10.030
Qi T, Weng J, Yu F, Zhang W et al (2021) Insights into the role of magnesium ions in affecting osteogenic differentiation of mesenchymal stem cells. Biol Trace Elem Res 199:559–567. https://doi.org/10.1007/s12011-020-02183-y
Hurle K, Oliveira JM, Reis RL, Pina S, Goetz-Neunhoeffer F (2021) Ion-doped brushite cements for bone regeneration. Acta Biomater 123:51–71. https://doi.org/10.1016/j.actbio.2021.01.004
Bose S, Fielding G, Tarafder S, Bandyopadhyay A (2013) Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends biotechnol 31:594–605. https://doi.org/10.1016/j.tibtech.2013.06.005
Gu Z, Xie H, Huang C, Peng H et al (2014) Effects of strontium-doped calcium polyphosphate on angiogenic growth factors expression of co-culturing system in vitro and of host cell in vivo. RSC Adv 4:2783–2792. https://doi.org/10.1039/C3RA44323J
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660. https://doi.org/10.1038/nm0603-653
Kulanthaivel S, Mishra U, Agarwal T et al (2015) Improved osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram Int 41:11323–11333. https://doi.org/10.1016/j.ceramint.2015.05.090
Xiao S, Wang M, Wang L, Zhu Y (2018) Environment-friendly synthesis of trace element Zn, Sr, and F codoping hydroxyapatite with non-cytotoxicity and improved osteoblast proliferation and differentiation. Biol Trace Elem Res 185:148–161. https://doi.org/10.1007/s12011-017-1226-5
Zima A, Czechowska J, Siek D, Ślósarczyk A (2017) Study on the new bone cement based on calcium sulfate and Mg, CO3 doped hydroxyapatite. Ceram Int 43:16196–16203
Ginebra MP, Canal C, Espanol M, Pastorino D, Montufar EB (2012) Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev 64:1090–1110. https://doi.org/10.1016/j.addr.2012.01.008
Aryaei A, Liu J, Jayatissa AH, Jayasuriya AC (2015) Cross-linked chitosan improves the mechanical properties of calcium phosphate-chitosan cement. Mater Sci Eng C Mater Biol Appl 54:14–19. https://doi.org/10.1016/j.msec.2015.04.024
Li Z, Yubao L, Yi Z, Lan W, Jansen JA (2010) In vitro and in vivo evaluation on the bioactivity of ZnO containing nano-hydroxyapatite/chitosan cement. J Biomed Mater Res A 93:269–279. https://doi.org/10.1002/jbm.a.32500
Czechowska ŚAJ, Zima PZA (2010) New bone implant material with calcium sulfate and Ti modified hydroxyapatite. J Achiev Mater Manuf Eng 43:170–177
Czechowska J, Zima A, Siek D, Ślósarczyk A (2016) The importance of chitosan and nano-TiHA in cement-type composites on the basis of calcium sulfate. Ceram Int 42:15559–15567. https://doi.org/10.1016/j.ceramint.2016.07.003
Zima A, Paszkiewicz Z, Siek D, Czechowska J et al (2012) Study on the new bone cement based on calcium sulfate and Mg, CO3 doped hydroxyapatite. Cera Int 38:4935–4942. https://doi.org/10.1016/j.ceramint.2012.02.086
Konishi T, Honda M, Nagaya M et al (2017) Injectable chelate-setting hydroxyapatite cement prepared by using chitosan solution: fabrication, material properties, biocompatibility, and osteoconductivity. J Biomater Appl 31:1319–1327. https://doi.org/10.1177/0885328217704060
Qi X, Li H, Qiao B, Li W et al (2013) Development and characterization of an injectable cement of nano calcium-deficient hydroxyapatite/multi(amino acid) copolymer/calcium sulfate hemihydrate for bone repair. Int J Nanomed 8:4441–4452. https://doi.org/10.2147/ijn.s54289
Idowu B, Cama G, Deb S, Di Silvio L (2014) In vitro osteoinductive potential of porous monetite for bone tissue engineering. J Tissue Eng 5:2041731414536572. https://doi.org/10.1177/2041731414536572
Sheikh Z, Abdallah MN, Al-Jaf F, Chen G et al (2020) Achieving enhanced bone regeneration using monetite granules with bone anabolic drug conjugates (C3 and C6) in rat mandibular defects. J Biomed Mater Res B Appl Biomater 108:2670–2680. https://doi.org/10.1002/jbm.b.34598
Boroujeni NM, Zhou H, Luchini TJ, Bhaduri SB (2014) Development of monetite/phosphorylated chitosan composite bone cement. J Biomed Mater Res B Appl Biomater 102:260–266. https://doi.org/10.1002/jbm.b.33003
Koju N, Sikder P, Gaihre B, Bhaduri SB (2018) Smart injectable self-setting monetite based bioceramics for orthopedic applications. Materials (Basel) 11:1258. https://doi.org/10.3390/ma11071258
Zhou H, Luchini TJ, Agarwal AK et al (2014) Development of monetite-nanosilica bone cement: a preliminary study. J Biomed Mater Res B Appl Biomater 102:1620–1626. https://doi.org/10.1002/jbm.b.33149
Arkin VH, Narendrakumar U, Madhyastha H, Manjubala I (2021) Cement doped with magnesium and strontium. ACS Omega 6:2477–2486. https://doi.org/10.1021/acsomega.0c03927
Bigi A, Bracci B, Panzavolta S (2004) Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials 25:2893–2899. https://doi.org/10.1016/j.biomaterials.2003.09.059
Liu W, Zhang J, Weiss P et al (2013) The influence of different cellulose ethers on both the handling and mechanical properties of calcium phosphate cements for bone substitution. Acta Biomater 9:5740–5750. https://doi.org/10.1016/j.actbio.2012.11.020
Taha A, Akram M, Jawad Z et al (2017) Strontium doped injectable bone cement for potential drug delivery applications. Mater Sci Eng C Mater Biol Appl 80:93–101. https://doi.org/10.1016/j.msec.2017.05.117
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27:2907–2915. https://doi.org/10.1016/j.biomaterials.2006.01.017
Lee GH, Makkar P, Paul K, Lee B (2017) Incorporation of BMP-2 loaded collagen conjugated BCP granules in calcium phosphate cement based injectable bone substitutes for improved bone regeneration. Mater Sci Eng C Mater Biol Appl 77:713–724. https://doi.org/10.1016/j.msec.2017.03.296
Liu J, Li J, Ye J (2016) Properties and cytocompatibility of anti-washout calcium phosphate cement by introducing locust bean gum. J Mater Sci Technol 32:1021–1026. https://doi.org/10.1016/j.jmst.2016.05.011
Dias AM, da Silva FG, Monteiro APD et al (2019) Polycaprolactone nanofibers loaded oxytetracycline hydrochloride and zinc oxide for treatment of periodontal disease. Mater Sci Eng C Mater Biol Appl 103:109798. https://doi.org/10.1016/j.msec.2019.109798
Letchmanan K, Shen SC, Ng WK, Kingshuk P et al (2017) Mechanical properties and antibiotic release characteristics of poly(methyl methacrylate)-based bone cement formulated with mesoporous silica nanoparticles. J Mech Behav Biomed Mater 72:163–170. https://doi.org/10.1016/j.jmbbm.2017.05.003
Alkhraisat MH, Cabrejos-Azama J, Rodríguez CR et al (2013) Magnesium substitution in brushite cements. Mater Sci Eng C Mater Biol Appl 33:475–481. https://doi.org/10.1016/j.msec.2012.09.017
Elahpour N, Rabiee SM, Ebrahimzadeh MH, Moradi A (2018) In-vitro formation and growth kinetics of apatite on a new light-cured composite calcium phosphate cement. Ceram Int 44:15317–15322. https://doi.org/10.1016/j.ceramint.2018.05.178
Lu J, Wei J, Yan Y et al (2011) Preparation and preliminary cytocompatibility of magnesium doped apatite cement with degradability for bone regeneration. J Mater Sci Mater Med 22:607–615. https://doi.org/10.1007/s10856-011-4228-4
Duan X, Liao HX, Zou HZ et al (2018) An injectable, biodegradable calcium phosphate cement containing poly lactic-co-glycolic acid as a bone substitute in ex vivo human vertebral compression fracture and rabbit bone defect models. Connect Tissue Res 59:55–65. https://doi.org/10.1080/03008207.2017.1301932
Lei X, Gao J, Xing F et al (2019) Comparative evaluation of the physicochemical properties of nano-hydroxyapatite/collagen and natural bone ceramic/collagen scaffolds and their osteogenesis-promoting effect on MC3T3-E1 cells. Regen Biomater 6:361–371. https://doi.org/10.1093/rb/rbz026
Danilchenko S, Kalinkevich O, Kuznetsov V et al (2010) Thermal transformations of the mineral component of composite biomaterials based on chitosan and apatite. Cryst Res Tech 45:685–691. https://doi.org/10.1002/crat.201000163
Miyazaki T, Sivaprakasam K, Tantry J, Suryanarayanan R (2009) Physical characterization of dibasic calcium phosphate dihydrate and anhydrate. J Pharm Sci 98:905–916. https://doi.org/10.1002/jps.21443
Sayyar S, Murray E, Thompson BC et al (2015) Processable conducting graphene/chitosan hydrogels for tissue engineering. J Mater Chem B 3:481–490. https://doi.org/10.1039/C4TB01636J
Al-Wafi R, Jafer R, Yahia IS et al (2017) Fast and easy synthesis of novel strontium apatite nanostructured phase: structure, spectroscopy, and dielectric analysis. Ceram Int 43(2017):17153–17159. https://doi.org/10.1016/j.ceramint.2017.09.137
Singh YP, Dasgupta S, Bhaskar R (2019) Preparation, characterization and bioactivities of nano anhydrous calcium phosphate added gelatin-chitosan scaffolds for bone tissue engineering. J Biomater Sci Polym Ed 30:1756–1778. https://doi.org/10.1080/09205063.2019.1663474
Sukhodub L, Sukhodub L, Chorna I (2016) Chitosan-apatite composites: synthesis and properties. Biopolym Cell 32:83–97. https://doi.org/10.7124/bc.000910
Dorozhkin S (2011) Self-setting calcium orthophosphate formulations: cements, concretes, pastes and putties. Int J Mater Chem 1:1–48. https://doi.org/10.5923/j.ijmc.20110101.01
Wu F, Wei J, Guo H et al (2008) Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater 4:1873–1884. https://doi.org/10.1016/j.actbio.2008.06.020
Zhang Z, Lai Q, Li Y et al (2017) Acidic pH environment induces autophagy in osteoblasts. Sci Rep 7:46161. https://doi.org/10.1038/srep46161
Kruse CR, Singh M, Targosinski S et al (2017) I. Sinha, J.A. Sørensen, E. Eriksson, K Nuutila, The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: in vitro and in vivo study. Wound Repair Regen 25:260–269. https://doi.org/10.1111/wrr.12526
Galow AM, Rebl A, Koczan D et al (2017) Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem Biophys Rep 10:17–25. https://doi.org/10.13140/RG.2.1.3770.3287
Zhou ZQ, Ye DP, Liang WG et al (2015) Preparation and characterization of a novel injectable strontium-containing calcium phosphate cement with collagen. Chin J Traumatol 18:33–38. https://doi.org/10.1016/j.matlet.2015.04.116
Chiang TY, Ho CC, Chen D et al (2010) Physicochemical properties and biocompatibility of chitosan oligosaccharide/gelatin/calcium phosphate hybrid cements. Mater Chem Phys 120:282–288. https://doi.org/10.1016/j.matchemphys.2009.11.007
Ye JR, Chen L, Zhang Y et al (2014) Turning the chitosan surface from hydrophilic to hydrophobic by layer-by-layer electro-assembly. RSC Adv 4:58200–58203. https://doi.org/10.1039/c4ra10327k
Laguta IV, Stavinskaya ON, Kuzema PA et al (2017) Hybrid materials on the basis of gelatin and hydrophilic–hydrophobic silica. Prot Met Phys Chem Surf 53:807–811. https://doi.org/10.1134/S2070205117050100
Moraleda B, San RJ, Rodriguez-Lorenzo L (2013) Influence of surface features of hydroxyapatite on the adsorption of proteins relevant to bone regeneration. J Biomed Mater Res A 101A:2332–2339. https://doi.org/10.1002/jbm.a.34528
Nakamura M, Nagai A, Yamashita K (2011) Improved wettability increases osteoblastic adhesion on hydroxyapatite. Phosphorus Res Bull 25:28–32. https://doi.org/10.3363/prb.25.28
Zhang J, Shi HS, Liu JQ et al (2015) Good hydration and cell-biological performances of superparamagnetic calcium phosphate cement with concentration-dependent osteogenesis and angiogenesis induced by ferric iron. J Mater Chem B 3:8782–8795. https://doi.org/10.1039/c5tb01440a
Glenske K, Donkiewicz P, Köwitsch A et al (2018) Applications of metals for bone regeneration. Int J Mol Sci 19:826. https://doi.org/10.3390/ijms19030826
Masaeli R, Jafarzadeh Kashi TS, Dinarvand R et al (2016) Efficacy of the biomaterials 3wt%-nanostrontium-hydroxyapatite-enhanced calcium phosphate cement (nanoSr-CPC) and nanoSr-CPC-incorporated simvastatin-loaded poly(lactic-co-glycolic-acid) microspheres in osteogenesis improvement: an explorative multi-phase experimental in vitro/vivo study. Mater Sci Eng C Mater Biol Appl 69:171–183. https://doi.org/10.1016/j.msec.2016.06.033
González-Vázquez A, Planell JA, Engel E (2014) Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater 10:2824–2833. https://doi.org/10.1016/j.actbio.2014.02.004
Hu F, Pan L, Zhang K et al (2014) (2014) Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts. PLoS ONE 9:e107217. https://doi.org/10.1371/journal.pone.0107217
Dvorak MM, Siddiqua A, Ward DT et al (2004) Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA 101:5140–5145. https://doi.org/10.1073/pnas.0306141101
Wagner AS, Glenske K, Wolf V et al (2017) Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Ann Anat 209:18–24. https://doi.org/10.1016/j.aanat.2016.09.005
Barbara A, Delannoy P, Denis BG, Marie PJ (2004) Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 53:532–537. https://doi.org/10.1016/j.metabol.2003.10.022
Braux J, Velard F, Guillaume C et al (2011) A new insight into the dissociating effect of strontium on bone resorption and formation. Acta Biomater 7(2011):2593–2603. https://doi.org/10.1016/j.actbio.2011.02.013
He LY, Zhang XM, Liu B et al (2016) Effect of magnesium ion on human osteoblast activity. Braz J Med Biol Res 49:e5257. https://doi.org/10.1590/1414-431X20165257
Leidi M, Dellera F, Mariotti M, Maier J (2011) High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res 24:1–6. https://doi.org/10.1684/mrh.2011.0271
Maradze D, Musson D, Zheng Y et al (2018) High magnesium corrosion rate has an effect on osteoclast and mesenchymal stem cell role during bone remodelling. Sci Rep 8:10003. https://doi.org/10.1038/s41598-018-28476-w
O’Connor JP, Kanjilal D, Teitelbaum M et al (2020) Zinc as a therapeutic agent in bone regeneration. Materials (Basel) 13:2211. https://doi.org/10.3390/ma13102211
Khatua C, Sengupta S, Kundu B et al (2019) Enhanced strength, in vitro bone cell differentiation and mineralization of injectable bone cement reinforced with multiferroic particles. Mater Des 167:107628. https://doi.org/10.1016/j.matdes.2019.107628
Lei Y, Xu Z, Ke Q et al (2017) Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 72:134–142. https://doi.org/10.1016/j.msec.2016.11.063
Pitz Hda S, Pereira A, Blasius MB et al (2016) In vitro evaluation of the antioxidant activity and wound healing properties of Jaboticaba (Plinia peruviana) fruit peel hydroalcoholic extract. Oxid Med Cell Longev 2016:3403586. https://doi.org/10.1155/2016/3403586
Liu W, Zhang G, Wu J et al (2020) Insights into the angiogenic effects of nanomaterials: mechanisms involved and potential applications. J Nanobiotechnology 18:9. https://doi.org/10.1186/s12951-019-0570-3
Zhu H, Guo D, Qi W, Xu K (2017) Development of Sr-incorporated biphasic calcium phosphate bone cement. Biomed Mater 12:015016. https://doi.org/10.1088/1748-605x/12/1/015016
Shu Y, Qiu F, Zhang Y (2017) Novel vaterite-containing tricalcium silicate bone cement by surface functionalization using 3-aminopropyltriethoxysilane: setting behavior, in vitro bioactivity and cytocompatibility. Biomed Mater 12:065007. https://doi.org/10.1088/1748-605X/aa84b8
Geng Z, Wang X, Zhao J, Li Z, Ma L, Zhu S (2018) The synergistic effect of strontium, bioactive glass and nano-hydroxyapatite promotes bone regeneration of critical-sized radial bone defects. Biomater Sci 6:2694–2703
Geng Z, Li X, Ji L, Li Z, Zhu S, Cui Z et al (2021) A novel snail-inspired bionic design of titanium with strontium-substituted hydroxyapatite coating for promoting osseointegration. J Mat Sci Tech 79:35–45. https://doi.org/10.1016/j.jmst.2020.11.041
Geng Z, Ji L, Li Z, Wang J, He H, Cui Z et al (2021) Nano-needle strontium-substituted apatite coating enhances osteoporotic osseointegration through promoting osteogenesis and inhibiting osteoclastogenesis. Bioact Mat 6:905–915. https://doi.org/10.1016/j.bioactmat.2020.09.024
Geng Z, Sang S, Wang S, Meng F, Li Z, Zhu S et al (2022) Optimizing the strontium content to achieve an ideal osseointegration through balancing apatite-forming ability and osteogenic activity. Biomat Adv 133:112647. https://doi.org/10.1016/j.msec.2022.112647
Nascimento M, Pelegrino M, Pieretti J, Seabra A (2020) How can nitric oxide help osteogenesis? AIMS Mol Sci 7:29–48. https://doi.org/10.3934/molsci.2020003
Tai YT, Cherng YG, Chang CC et al (2007) Pretreatment with low nitric oxide protects osteoblasts from high nitric oxide-induced apoptotic insults through regulation of c-Jun N-terminal kinase/c-Jun-mediated Bcl-2 gene expression and protein translocation. J Orthop Res 25:625–635. https://doi.org/10.1002/jor.20365
Kim YH, Choi EM (2009) Stimulation of osteoblastic differentiation and inhibition of interleukin-6 and nitric oxide in MC3T3-E1 cells by pomegranate ethanol extract. Phytother Res 23:737–739. https://doi.org/10.1002/ptr.2587
Zhong X, Xiu LL, Wei GH et al (2011) Bezafibrate enhances proliferation and differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS activation. Acta Pharmacol Sin 32:591–600. https://doi.org/10.1038/aps.2011.15
Acknowledgements
The authors would like to express them acknowledge to the Brazilian agencies and Center of Microscopy at UFMG.
Funding
The authors have received individual sources of funding that have supported the work from the Brazilian agencies National Scientific and Technological Development Council (CNPq), Higher Education Personnel Improvement Coordination (CAPES), and Research Support Foundation of the State of Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
Alexa M Dias and Maria E Cortés were responsible for formulating the research question, designing, and conducting the study, analyzed the data acquisition, and wrote the main manuscript text; Isabela do N Canhas, Carlos G Bruziquesi, Marcelo G Speziali, and Rubén D Sinisterra were responsible for conducting parts of the study and analyzed the data acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research is in accord with the ethics principles.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, A.M., do Nascimento Canhas, I., Bruziquesi, C.G.O. et al. Magnesium (Mg2 +), Strontium (Sr2 +), and Zinc (Zn2 +) Co-substituted Bone Cements Based on Nano-hydroxyapatite/Monetite for Bone Regeneration. Biol Trace Elem Res 201, 2963–2981 (2023). https://doi.org/10.1007/s12011-022-03382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03382-5