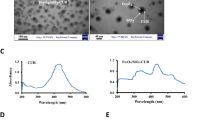
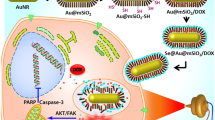
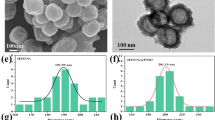
Abstract
In cancer treatment, the complexity of tumors seriously affects the therapeutic potential of the treatment. Treatments with combination therapy result in more potent effects than monotherapy or their theoretical combination in cancer treatment. Photothermal therapy (PTT) includes applying phototherapeutic agents that cause local hyperthermia responsible for the thermal ablation of tumor cells after applying near-infrared light and is often applied with other combination therapies. In this study, the chemo-PTT potential of synthesized drug-loaded and targeted GEM/TRA-MC@Si nanocomposite on Her2 positive breast cancer cell line (SK-BR-3) and human triple-negative breast cancer cell line (MDA-MB-231) was investigated using NIR application as in vitro. First, the cell viability (IC50) value of the GEM/TRA-MC@Si nanocomposite was determined as 25 µg/µL. Then, chemo-PTT was performed, and the viability of the cells was evaluated. In addition, the live/dead cell rate was established by staining with the Calcein-AM and EthD-1, and apoptosis tests were completed. When the surface temperature of Her2 positive SK-BR-3 cells exceeded 47 °C during PTT with an irradiation time of > 100 s, it caused cell death. In this study, it was demonstrated that in vitro PTT (1 W/cm2, 180 s) was applied using GEM/TRA-MC@Si nanocomposite (25 µg/mL) on her2 + SK-BR-3 cell line, which contributed to the reduction of cell viability. In addition, this study demonstrates that chemo-PTT with targeted GEM/TRA-MC@Si nanocomposite induced SK-BR-3 cell viability and can initiate cell death through the apoptosis pathway under optimized irradiation conditions. Herewith chemo-PTT combination therapy of targeted GEM-TRA/MC@Si nanocomposite was found to be effective on SK-BR-3 cells as in vitro.








Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Xu, Z., Zhang, Y., Zhou, W., Wang, L., Xu, G., Ma, M., … Yang, C. (2021). NIR-II-activated biocompatible hollow nanocarbons for cancer photothermal therapy. Journal of Nanobiotechnology, 19(1), 137. https://doi.org/10.1186/s12951-021-00884-7
Zhang, Y., Leonard, M., Shu, Y., Yang, Y., Shu, D., Guo, P., & Zhang, X. (2017). Overcoming tamoxifen resistance of human breast cancer by targeted gene silencing using multifunctional pRNA nanoparticles. ACS Nano, 11(1), 335–346. https://doi.org/10.1021/acsnano.6b05910
Huang, H., & Lovell, J. F. (2017). Advanced functional nanomaterials for theranostics. Advanced Functional Materials, 27(2), 1603524. https://doi.org/10.1002/adfm.201603524
Poudel, B. K., Gupta, B., Ramasamy, T., Thapa, R. K., Pathak, S., Oh, K. T., … Kim, J. O. (2017). PEGylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids and Surfaces B: Biointerfaces, 160, 73–83. https://doi.org/10.1016/j.colsurfb.2017.09.010
Shen, S., Liu, M., Li, T., Lin, S., & Mo, R. (2017). Recent progress in nanomedicine-based combination cancer therapy using a site-specific co-delivery strategy. Biomaterials Science, 5(8), 1367–1381. https://doi.org/10.1039/c7bm00297a
Li, X., Liu, C., Wang, S., Jiao, J., Di, D., Jiang, T., … Wang, S. (2017). Poly(acrylic acid) conjugated hollow mesoporous carbon as a dual-stimuli triggered drug delivery system for chemo-photothermal synergistic therapy. Materials Science and Engineering C, 71, 594–603. https://doi.org/10.1016/j.msec.2016.10.037
Zhao, Q., Wang, X., Yan, Y., Wang, D., Zhang, Y., Jiang, T., & Wang, S. (2017). The advantage of hollow mesoporous carbon as a near-infrared absorbing drug carrier in chemo-photothermal therapy compared with IR-820. European Journal of Pharmaceutical Sciences, 99, 66–74. https://doi.org/10.1016/j.ejps.2016.11.031
Lu, H., Zhao, Q., Wang, X., Mao, Y., Chen, C., Gao, Y., … Wang, S. (2020). Multi-stimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids and Surfaces B: Biointerfaces, 190, 110941. https://doi.org/10.1016/j.colsurfb.2020.110941
Li, X., Yan, Y., Lin, Y., Jiao, J., Wang, D., Di, D., … Wang, S. (2017). Hollow mesoporous carbon as a near-infrared absorbing carrier compared with mesoporous carbon nanoparticles for chemo-photothermal therapy. Journal of Colloid and Interface Science, 494, 159–169. https://doi.org/10.1016/j.jcis.2017.01.090
Feng, S., Lu, J., Wang, K., Di, D., Shi, Z., Zhao, Q., & Wang, S. (2022). Advances in smart mesoporous carbon nanoplatforms for photothermal–enhanced synergistic cancer therapy. Chemical Engineering Journal, 435, 134886. https://doi.org/10.1016/j.cej.2022.134886
Wang, Y., Zhang, H., Xie, J., Liu, Y., Wang, S., & Zhao, Q. (2020). Three dimensional mesoporous carbon nanospheres as carriers for chemo-photothermal therapy compared with two dimensional graphene oxide nanosheets. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 590, 124498. https://doi.org/10.1016/j.colsurfa.2020.124498
Zhang, Y., Zhu, J., Huang, G., Zhu, J., & He, D. (2020). Potential applications of multifunctional mesoporous carbon nanoplatform for tumor microenvironment improving by combined chemo-/phototherapy. Carbon, 163, 128–136. https://doi.org/10.1016/j.carbon.2020.02.029
Zhou, M., Zhao, Q., Wu, Y., Feng, S., Wang, D., Zhang, Y., & Wang, S. (2020). Mesoporous carbon nanoparticles as multi-functional carriers for cancer therapy compared with mesoporous silica nanoparticles. An Official Journal of the American Association of Pharmaceutical Scientists, 21(2), 42. https://doi.org/10.1208/s12249-019-1604-8
Li, Y., Xiao, B. X., Dong, J., Liu, Y., Gao, S., Pang, J., & Sun, Z. (2018). Near-infrared light-responsive nanoparticles for improved anticancer efficacy through synergistic chemo-photothermal therapy. Pharmaceutical Development and Technology, 23(1), 116–124. https://doi.org/10.1080/10837450.2017.1402934
Yang, K., Feng, L., & Liu, Z. (2016). Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Advanced Drug Delivery Reviews, 105(Pt B), 228–241. https://doi.org/10.1016/j.addr.2016.05.015
Wang, X., Liu, Y., Liu, Z., Hu, J., Guo, H., & Wang, F. (2018). Synergistic chemo-photothermal therapy of tumor by hollow carbon nanospheres. Biochemical and Biophysical Research Communications, 495(1), 867–872. https://doi.org/10.1016/j.bbrc.2017.11.130
Wang, J., & Li, N. (2017). Functional hollow nanostructures for imaging and phototherapy of tumors. Journal of Materials Chemistry B, 5(43), 8430–8445. https://doi.org/10.1039/c7tb02381b
Lim, W. Q., Phua, S. Z. F., Xu, H. V., Sreejith, S., & Zhao, Y. (2016). Recent advances in multifunctional silica-based hybrid nanocarriers for bioimaging and cancer therapy. Nanoscale, 8(25), 12510–12519. https://doi.org/10.1039/c5nr07853a
Park, W., Cho, S., Han, J., Shin, H., Na, K., Lee, B., & Kim, D. H. (2018). Advanced smart-photosensitizers for more effective cancer treatment. Biomaterials Science, 6(1), 79–90. https://doi.org/10.1039/c7bm00872d
Petrilli, R., Pinheiro, D. P., de Cássia Evangelista de Oliveira, F., Galvão, G. F., Marques, L. G. A., Lopez, R. F. V., … Eloy, J. O. (2021). Immunoconjugates for cancer targeting: A review of antibody-drug conjugates and antibody-functionalized nanoparticles. Current medicinal chemistry, 28(13), 2485–2520. https://doi.org/10.2174/0929867327666200525161359
Wathoni, N., Puluhulawa, L. E., Joni, I. M., Muchtaridi, M., Mohammed, A. F. A., Elamin, K. M., … Gozali, D. (2022). Monoclonal antibody as a targeting mediator for nanoparticle targeted delivery system for lung cancer. Drug Delivery, 29(1), 2959–2970. https://doi.org/10.1080/10717544.2022.2120566
Kirpotin, D., Park, J. W., Hong, K., Zalipsky, S., Li, W. L., Carter, P., … Papahadjopoulos, D. (1997). Sterically stabilized anti-HER2 immunoliposomes: Design and targeting to human breast cancer cells in vitro. Biochemistry, 36(1), 66–75. https://doi.org/10.1021/bi962148u
Xu, R., Sui, J., Zhao, M., Yang, Y., Tong, L., Liu, Y., … Zhang, X. (2022). Targeted inhibition of HER-2 positive breast cancer cells by trastuzumab functionalized pullulan-doxorubicin nanoparticles. Polymer Testing, 113, 107669. https://doi.org/10.1016/j.polymertesting.2022.107669
Mozafarinia, M., Karimi, S., Farrokhnia, M., & Esfandiari, J. (2021). In vitro breast cancer targeting using Trastuzumab-conjugated mesoporous silica nanoparticles: Towards the new strategy for decreasing size and high drug loading capacity for drug delivery purposes in MSN synthesis. Microporous and Mesoporous Materials, 316, 110950. https://doi.org/10.1016/j.micromeso.2021.110950
Catala, A., Dzieciatkowska, M., Wang, G., Gutierrez-Hartmann, A., Simberg, D., Hansen, K. C., … Catalano, C. E. (2021). Targeted ıntracellular delivery of trastuzumab using designer phage lambda nanoparticles alters cellular programs in human breast cancer cells. ACS Nano, 15(7), 11789–11805. https://doi.org/10.1021/acsnano.1c02864
Zhang, X., Liu, J., Li, X., Li, F., Lee, R. J., Sun, F., … Teng, L. (2019). Trastuzumab-coated nanoparticles loaded with docetaxel for breast cancer therapy. Dose-response : a publication of International Hormesis Society, 17(3), 1559325819872583–1559325819872583. https://doi.org/10.1177/1559325819872583
Niza, E., Noblejas-López, M. D. M., Bravo, I., Nieto-Jiménez, C., Castro-Osma, J. A., Canales-Vázquez, J., … Alonso-Moreno, C. (2019). Trastuzumab-targeted biodegradable nanoparticles for enhanced delivery of dasatinib in HER2+ metastasic breast cancer. Nanomaterials (Basel, Switzerland), 9(12), 1793. https://doi.org/10.3390/nano9121793
Wang, Y., Wang, K., Zhang, R., Liu, X., Yan, X., Wang, J., … Huang, R. (2014). Synthesis of core-shell graphitic carbon@silica nanospheres with dual-ordered mesopores for cancer-targeted photothermochemotherapy. ACS Nano, 8(8), 7870–7879. https://doi.org/10.1021/nn5027214
Quan, G., Pan, X., Wang, Z., Wu, Q., Li, G., Dian, L., … Wu, C. (2015). Lactosaminated mesoporous silica nanoparticles for asialoglycoprotein receptor targeted anticancer drug delivery. Journal of Nanobiotechnology (Vol. 13). https://doi.org/10.1186/s12951-015-0068-6
Freitas, L. B. de O., Corgosinho, L. de M., Faria, J. A. Q. A., dos Santos, V. M., Resende, J. M., Leal, A. S., … Sousa, E. M. B. de. (2017). Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous and Mesoporous Materials, 242, 271–283. https://doi.org/10.1016/j.micromeso.2017.01.036
Meng, H., Liong, M., Xia, T., Li, Z., Ji, Z., Zink, J. I., & Nel, A. E. (2010). Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and p-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano, 4(8), 4539–4550. https://doi.org/10.1021/nn100690m
Yu, K., Zhao, J., Zhang, Z., Gao, Y., Zhou, Y., Teng, L., & Li, Y. (2016). Enhanced delivery of Paclitaxel using electrostatically-conjugated Herceptin-bearing PEI/PLGA nanoparticles against HER-positive breast cancer cells. International Journal of Pharmaceutics, 497(1–2), 78–87. https://doi.org/10.1016/j.ijpharm.2015.11.033
Papa, A. L., Sidiqui, A., Balasubramanian, S. U. A., Sarangi, S., Luchette, M., Sengupta, S., & Harfouche, R. (2013). PEGylated liposomal Gemcitabine: Insights into a potential breast cancer therapeutic. Cellular Oncology, 36(6), 449–457. https://doi.org/10.1007/s13402-013-0146-4
Zhao, R., Han, X., Li, Y., Wang, H., Ji, T., Zhao, Y., & Nie, G. (2017). Photothermal effect enhanced cascade-targeting strategy for ımproved pancreatic cancer therapy by gold nanoshell@mesoporous silica nanorod. ACS Nano, 11(8), 8103–8113. https://doi.org/10.1021/acsnano.7b02918
Zhang, X., Wu, J., Williams, G. R., Niu, S., Qian, Q., & Zhu, L. M. (2019). Functionalized MoS 2 -nanosheets for targeted drug delivery and chemo-photothermal therapy. Colloids and Surfaces B: Biointerfaces, 173, 101–108. https://doi.org/10.1016/j.colsurfb.2018.09.048
Kaur, T., Kaur, S., & Kaur, P. (2017). Development and validation of UV-spectrophotometric methods for determination of gemcitabine hydrochloride in bulk and polymeric nanoparticles. International Journal of Applied Pharmaceutics, 9(5), 60–65. https://doi.org/10.22159/ijap.2017v9i5.19726
Ariyasu, S., Mu, J., Zhang, X., Huang, Y., Yeow, E. K. L., Zhang, H., & Xing, B. (2017). Investigation of thermally ınduced cellular ablation and heat response triggered by planar MoS2-based nanocomposite. Bioconjugate Chemistry, 28(4), 1059–1067. https://doi.org/10.1021/acs.bioconjchem.6b00741
Geretto, M., Ponassi, M., Casale, M., Pulliero, A., Cafeo, G., Malagreca, F., … Izzotti, A. (2018). A novel calix[4]pyrrole derivative as a potential anticancer agent that forms genotoxic adducts with DNA. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-29314-9
Fang, Y., Zheng, G., Yang, J., Tang, H., Zhang, Y., Kong, B., … Zhao, D. (2014). Dual-pore mesoporous carbon@silica composite core-shell nanospheres for multidrug delivery. Angewandte Chemie - International Edition, 53(21), 5366–5370. https://doi.org/10.1002/anie.201402002
Fang, Y., Gu, D., Zou, Y., Wu, Z., Li, F., Che, R., … Zhao, D. (2010). A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angewandte Chemie - International Edition, 49(43), 7987–7991. https://doi.org/10.1002/anie.201002849
Wang, Y., Santos, A., Evdokiou, A., & Losic, D. (2015). An overview of nanotoxicity and nanomedicine research: Principles, progress and implications for cancer therapy. Journal of Materials Chemistry B, 3(36), 7153–7172. https://doi.org/10.1039/c5tb00956a
Meng, H., Wang, M., Liu, H., Liu, X., Situ, A., Wu, B., … Nel, A. E. (2015). Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gmcitabine and pclitaxel dlivery to hman pncreatic cncer in mice. ACS Nano, 9(4), 3540–3557. https://doi.org/10.1021/acsnano.5b00510
Malfanti, A., Miletto, I., Bottinelli, E., Zonari, D., Blandino, G., Berlier, G., & Arpicco, S. (2016). Delivery of gemcitabine prodrugs employing mesoporous silica nanoparticles. Molecules, 21(4), 522. https://doi.org/10.3390/molecules21040522
Patra, C. R., Bhattacharya, R., Wang, E., Katarya, A., Lau, J. S., Dutta, S., … Mukhopadhyay, D. (2008). Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Research, 68(6), 1970–1978. https://doi.org/10.1158/0008-5472.CAN-07-6102
Birhanu, G., Javar, H. A., Seyedjafari, E., & Zandi-Karimi, A. (2017). Nanotechnology for delivery of gemcitabine to treat pancreatic cancer. Biomedicine and Pharmacotherapy. Elsevier Masson SAS. https://doi.org/10.1016/j.biopha.2017.01.071
Han, H., Li, S., Zhong, Y., Huang, Y., Wang, K., Jin, Q., … Yao, K. (2022). Emerging pro-drug and nano-drug strategies for gemcitabine-based cancer therapy. Asian Journal of Pharmaceutical Sciences, 17(1), 35–52. https://doi.org/10.1016/j.ajps.2021.06.001
Zaharudin, N. S., Mohamed Isa, E. D., Ahmad, H., Abdul Rahman, M. B., & Jumbri, K. (2020). Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. Journal of Saudi Chemical Society, 24(3), 289–302. https://doi.org/10.1016/j.jscs.2020.01.003
Xue, Z., Zhang, F., Qin, D., Wang, Y., Zhang, J., Liu, J., … Lu, X. (2014). One-pot synthesis of silver nanoparticle catalysts supported on N-doped ordered mesoporous carbon and application in the detection of nitrobenzene. Carbon, 69, 481–489. https://doi.org/10.1016/j.carbon.2013.12.051
Melamed, J. R., Edelstein, R. S., & Day, E. S. (2015). Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano, 9(1), 6–11. https://doi.org/10.1021/acsnano.5b00021
Martin, S. J., Henry, C. M., & Cullen, S. P. (2012). A perspective on mammalian caspases as positive and negative regulators of ınflammation. Molecular Cell, 46(4), 387–397. https://doi.org/10.1016/j.molcel.2012.04.026
Li, J. L., & Gu, M. (2010). Surface plasmonic gold nanorods for enhanced two-photon microscopic imaging and apoptosis induction of cancer cells. Biomaterials, 31(36), 9492–9498. https://doi.org/10.1016/j.biomaterials.2010.08.068
Mocan, T., Matea, C. T., Cojocaru, I., Ilie, I., Tabaran, F. A., Zaharie, F., … Mocan, L. (2014). Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. Journal of Cancer, 5(8), 679–688. https://doi.org/10.7150/jca.9481
Tong, L., & Cheng, J. X. (2009). Gold nanorod-mediated photothermolysis induces apoptosis of macrophages via damage of mitochondria. Nanomedicine, 4(3), 265–276. https://doi.org/10.2217/nnm.09.4
Freeman, Starr, D. A., & O’connor. (2016). 乳鼠心肌提取 HHS public access. Physiology & behavior, 176(1), 139–148. https://doi.org/10.1016/j.biomaterials.2015.04.013.Single
Shih, E. C. (2015). PhD thesis, University of Nevada, Las Vegas, ABD.
Chen, W., Zeng, K., Liu, H., Ouyang, J., Wang, L., Liu, Y., … Liu, Y. N. (2017). Cell membrane camouflaged hollow Prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Advanced Functional Materials, 27(11). https://doi.org/10.1002/adfm.201605795
Su, J., Sun, H., Meng, Q., Yin, Q., Zhang, P., Zhang, Z., … Li, Y. (2016). Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Advanced Functional Materials, 26(41), 7495–7506. https://doi.org/10.1002/adfm.201603381
Yi, X., Yang, K., Liang, C., Zhong, X., Ning, P., Song, G., … Liu, Z. (2015). Imaging-guided combined photothermal and radiotherapy to treat subcutaneous and metastatic tumors using ıodine-131-doped copper sulfide nanoparticles. Advanced Functional Materials, 25(29), 4689–4699. https://doi.org/10.1002/adfm.201502003
Perez, R. A., Singh, R. K., Kim, T. H., & Kim, H. W. (2017). Silica-based multifunctional nanodelivery systems toward regenerative medicine. Materials Horizons, 4(5), 772–799. https://doi.org/10.1039/c7mh00017k
Acknowledgements
The authors kindly thank Fiber Last cooperation (Ankara/Turkey) for providing the laser device. Also, we are grateful to Ege University Planning and Monitoring Coordination of Organizational Development and the Directorate of Library and Documentation for their support in the editing and proofreading service of this study.
Funding
Financial support was provided by Ege University Scientific Research Projects (BAP) Coordinator ship (Project Number: FDK-2020–21380) and the Scientific and Technical Research Council of Turkey (TÜBİTAK) with 2211-C and 2214-A coded doctoral research support (grant number, respectively: 1649B031802269 and 1059B141801597).
Author information
Authors and Affiliations
Contributions
FY: writing—review and editing, resources, supervision, project administration, funding acquisition. AT: conceptualization, investigation, data curation, formal analysis, writing—original draft, validation, methodology, software.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tunçel, A., Yurt, F. Chemo-Photothermal Combination Therapy of HER-2 Overexpressing Breast Cancer Cells with Dual-Ordered Mesoporous Carbon@Silica Nanocomposite. Appl Biochem Biotechnol 195, 1904–1927 (2023). https://doi.org/10.1007/s12010-022-04235-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04235-6