Abstract
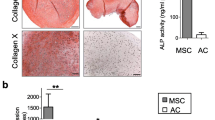
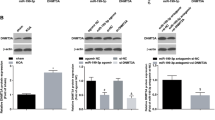
Cartilage hypertrophy is a condition in which the cells are completely differentiated, and new morphological changes and mineralization prevent proper cellular functions. The occurrence of hypertrophy during differentiation fails current regenerative strategies for treatment. Strategies to minimize hypertrophy in chondrocytes are categorized into two levels of protein and gene. Among these strategies, one way to affect multiple pathways involved in the development of hypertrophy is to manage microRNA activity in cells. Recent miRNA profiling studies have shown that miR-195-5p upregulates through the transition from chondrogenic to hypertrophic state. Bioinformatics assessment of microRNA targets also indicates that several genes repressed by miR-195-5p play important roles in processes related to hypertrophy. The aim of this study was to develop a microRNA Tough Decoy to suppress miR-195-5p and investigate whether it can prevent a hypertrophic state in chondrocytes. The Tough Decoy (TUD) was designed and evaluated bioinformatically and then cloned into the pLVX-Puro plasmid. The TUD function was validated by Dual-Luciferase assay and qRT-PCR. After delivering TUD to C28/I2 chondrocytes cultured in a hypertrophic medium, hypertrophic differentiation was assessed by histochemical staining, quantitative RT-PCR of hypertrophy marker genes, and alkaline phosphatase activity. Results showed that the TUD could inhibit miRNA efficiently and downregulate hypertrophic markers such as RUNX2, alkaline phosphatase, and collagen 10 significantly compared with the control group. Alcian blue and alizarin red staining also demonstrated the optimal effect of gene constructs on tissue properties and mineralization of the TUD group. Delivering the miR-195-5p Tough Decoy to the cartilage cells can prevent the occurrence of hypertrophy in chondrocytes and could be considered as a candidate for the treatment of other diseases such as osteoarthritis.



Similar content being viewed by others
References
Gauci, S. J., Golub, S. B., Tutolo, L., Little, C. B., Sims, N. A., Lee, E. R., Mackie, E. J., & Fosang, A. J. (2008). Modulating chondrocyte hypertrophy in growth plate and osteoarthritic cartilage. Journal of Musculoskeletal & Neuronal Interactions, 8(4), 308–310.
Sekiya, I., Vuoristo, J. T., Larson, B. L., & Prockop, D. J. (2002). In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proceedings of the National Academy of Sciences of the United States of America,99, 4397–4402.
Van der Kraan, P. M., & Van den Berg, W. B. (2012). Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage, 20(3), 223–232.
Cancedda, R., Dozin, B., Giannoni, P., & Quarto, R. (2003). Tissue engineering and cell therapy of cartilage and bone. Matrix Biology, 22(1), 81–91.
Koelling, S., & Miosge, N. (2009). Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opinion on Biological Therapy, 9(11), 1399–1405.
Şafak, A. S., Abdik, E. A., Abdik, H., Taşlı, P. N. and Şahin, F. (2019) A novel approach to septal perforation repair: septal cartilage cells induce chondrogenesis of hASCs in vitro. Applied biochemistry and biotechnology, 188, 942–951.
Nehrer, S., Domayer, S., Dorotka, R., Schatz, K., Bindreiter, U., & Kotz, R. (2006). Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. European Journal of Radiology, 57, 3–8.
Yang, L., Tsang, K. Y., Tang, H. C., Chan, D., & Cheah, K. S. (2014). Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of the National Academy of Sciences,111, 12097–12102.
Chen, S., Fu, P., Cong, R., Wu, H., & Pei, M. (2015). Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes & diseases, 2, 76–95.
Kouhkan, F., Hafizi, M., Mobarra, N., Mossahebi-Mohammadi, M., Mohammadi, S., Behmanesh, M., Zomorrod, M. S., Alizadeh, S., Lahmy, R., & Daliri, M. (2014). miRNAs: a new method for erythroid differentiation of hematopoietic stem cells without the presence of growth factors. Applied Biochemistry and Biotechnology, 172(4), 2055–2069.
Neshati, V., Mollazadeh, S., Bazzaz, B. S. F., De Vries, A. A., Mojarrad, M., Naderi-Meshkin, H., Neshati, Z., Mirahmadi, M., & Kerachian, M. A. (2018). MicroRNA-499a-5p promotes differentiation of human bone marrow-derived mesenchymal stem cells to cardiomyocytes. Applied Biochemistry and Biotechnology, 186(1), 245–255.
Barter, M. J., Tselepi, M., Gómez, R., Woods, S., Hui, W., Smith, G. R., Shanley, D. P., Clark, I. M., & Young, D. A. (2015). Genome-wide microRNA and gene analysis of mesenchymal stem cell chondrogenesis identifies an essential role and multiple targets for miR-140-5p. Stem Cells, 33, 3266–3280.
Gabler, J., Ruetze, M., Kynast, K. L., Grossner, T., Diederichs, S., & Richter, W. (2015). Stage-specific miRs in chondrocyte maturation: differentiation-dependent and hypertrophy-related miR clusters and the miR-181 family. Tissue Engineering Part A, 21, 2840–2851.
Mullokandov, G., Baccarini, A., Ruzo, A., Jayaprakash, A. D., Tung, N., Israelow, B., Evans, M. J., Sachidanandam, R., & Brown, B. D. (2012). High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nature Methods, 9, 840.
Rennie, W., Liu, C., Carmack, C. S., Wolenc, A., Kanoria, S., Lu, J., Long, D., & Ding, Y. (2014). STarMir: a web server for prediction of microRNA binding sites. Nucleic Acids Research, 42, W114–W118.
Vejnar, C. E., & Zdobnov, E. M. (2012). MiRmap: comprehensive prediction of microRNA target repression strength. Nucleic Acids Research, 40, 11673–11683.
Barta, T., Peskova, L., & Hampl, A. (2016). miRNAsong: a web-based tool for generation and testing of miRNA sponge constructs in silico. Scientific reports,6, 36625.
Denman, R. B. (1993). Using RNAFOLD to predict the activity of small catalytic RNAs. Biotechniques, 15, 1090–1095.
Gibcus, J. H., Tan, L. P., Harms, G., Schakel, R. N., de Jong, D., Blokzijl, T., Möller, P., Poppema, S., Kroesen, B.-J., & van den Berg, A. (2009). Hodgkin lymphoma cell lines are characterized by a specific miRNA expression profile. Neoplasia (New York, NY),11, 167.
Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitrochondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238(1), 265–272.
Ham, O., Song, B.-W., Lee, S.-Y., Choi, E., Cha, M.-J., Lee, C. Y., Park, J.-H., Kim, I.-K., Chang, W., & Lim, S. (2012). The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials, 33(18), 4500–4507.
Karlsen, T. A., Jakobsen, R. B., Mikkelsen, T. S., & Brinchmann, J. E. (2013). microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells and Development, 23, 290–304.
Mackay, A. M., Beck, S. C., Murphy, J. M., Barry, F. P., Chichester, C. O., & Pittenger, M. F. (1998). Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Engineering, 4, 415–428.
Peng, B., Chen, Y., & Leong, K. W. (2015). MicroRNA delivery for regenerative medicine. Advanced Drug Delivery Reviews, 88, 108–122.
Ebert, M. S., Neilson, J. R., & Sharp, P. A. (2007). MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature Methods, 4, 721.
Sun, L.-L., Li, W.-D., Lei, F.-R., & Li, X.-Q. (2018). The regulatory role of microRNAs in angiogenesis-related diseases. Journal of Cellular and Molecular Medicine, 22, 4568–4587.
Haraguchi, T., Ozaki, Y., & Iba, H. (2009). Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Research, 37, e43–e43.
Liu, L., Chen, L., Xu, Y., Li, R., & Du, X. (2010). microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochemical and Biophysical Research Communications, 400, 236–240.
Xu, T., Zhu, Y., Xiong, Y., Ge, Y. Y., Yun, J. P., & Zhuang, S. M. (2009). MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology, 50(1), 113–121.
Ebrahimi, A., Nikokar, I., Zokaei, M., & Bozorgzadeh, E. (2018). Design, development and evaluation of microRNA-199a-5p detecting electrochemical nanobiosensor with diagnostic application in triple negative breast cancer. Talanta, 189, 592–598.
Honardoost, M., & Rad, S. M. A. H. (2018). Triangle of AKT2, miRNA, and tumorigenesis in different cancers. Applied Biochemistry and Biotechnology, 185, 524–540.
Singh, R., Yadav, V., & Saini, N. (2015). MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Scientific Reports, 5, 17454.
Gu, Y. L., Rong, X. X., Wen, L. T., Zhu, G. X., & Qian, M. Q. (2017). miR-195 inhibits the proliferation and migration of chondrocytes by targeting GIT1. Molecular Medicine Reports, 15(1), 194–200.
Ito, K., Maruyama, Z., Sakai, A., Izumi, S., Moriishi, T., Yoshida, C., Miyazaki, T., Komori, H., Takada, K., & Kawaguchi, H. (2014). Overexpression of Cdk6 and Ccnd1 in chondrocytes inhibited chondrocyte maturation and caused p53-dependent apoptosis without enhancing proliferation. Oncogene, 33, 1862.
Zhang, M., Xie, R., Hou, W., Wang, B., Shen, R., Wang, X., Wang, Q., Zhu, T., Jonason, J. H., & Chen, D. (2009). PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. Journal of Cell Science, 122(Pt 9), 1382–1389.
Cao, X., Duan, Z., Yan, Z., Li, Y., Li, L., Sun, J., Han, P., Li, P., Wei, L., & Wei, X. (2019). miR-195 contributes to human osteoarthritis via targeting PTHrP. Journal of Bone and Mineral Metabolism, 37(4), 711–721.
Almeida, M. I., Silva, A. M., Vasconcelos, D. M., Almeida, C. R., Caires, H., Pinto, M. T., Calin, G. A., Santos, S. G., & Barbosa, M. A. (2016). miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget,7, 7.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This research was financially supported by the deputy of research, Guilan University of Medical Sciences, Iran (grant number: IR-96123).
Author information
Authors and Affiliations
Contributions
P.A.P.: performed experiments and co-wrote the paper. F.R.: performed experiments. M.H.R.: designed and performed experiments. SMT R.T.: co-wrote the paper. A.E.: designed and performed experiments, performed bioinformatics analyses, analyzed data, and co-wrote the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbasi Pashaki, P., Rahim, F., Habibi Roudkenar, M. et al. MicroRNA Tough Decoy Knockdowns miR-195 and Represses Hypertrophy in Chondrocytes. Appl Biochem Biotechnol 191, 1056–1071 (2020). https://doi.org/10.1007/s12010-020-03229-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03229-6