Abstract
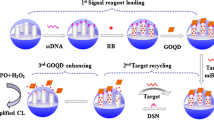
A simple, rapid response time and ultrahigh sensitive chemiluminescence (CL) DNA assay based on Fe3O4@SiO2@Au-functionalized magnetic nanoparticles (Au-MNPs) was developed for detection of p53 tumor suppressor gene. In this study, 2′,6′-dimethylcarbonylphenyl-10-sulfopropyl acridinium-9-carboxylate 4′-NHS ester (NSP-DMAE-NHS), as a new kind of highly efficient luminescence reagent, was immobilized on the complementary sequence of the wild-type p53 (ssDNA) to improve the detection sensitivity. The optimal concentration of ssDNA-(NSP-DMAE-NHS) conjugates mixed with the wild-type p53 (wtp53) samples respectively. Then, the wtp53-Au-MNPs conjugates were added to continue the competitive reaction in the above solution. Subsequently, the Au-MNPs separated under magnetic field, measured by a homemade luminescent measurement system. Under optimal conditions, the method exhibited ultrasensitive sensitivity with a detection limit of 0.001 ng mL−1 (0.16 pM), a wide range of liner response from 0.001 ng mL−1~6.6 μg mL−1. Therefore, the immunomagnetic nanocomposites-based detection strategy was rapid, low-cost, and highly sensitive that can be easily extended to the early diagnosis of cancer development and monitoring of patient therapy.






Similar content being viewed by others
References
Junttila, M. R., & Evan, G. I. (2009). p53— a Jack of all trades but master of none. Nature Reviews Cancer, 9(11), 821–829.
Evan, G. I., & Vousden, K. H. (2001). Proliferation, cell cycle and apoptosis in cancer. Nature, 411(6835), 342–348.
Duffy, M. J., Synnott, N. C., McGowan, P. M., Crown, J., O’Connor, D., & Gallagher, W. M. (2014). p53 as a target for the treatment of cancer. Cancer Treatment Reviews, 40(10), 1153–1160.
Gaspar, V. M., Correia, I. J., Sousa, A., Silva, F., Paquete, C. M., Queiroz, J. A., & Sousa, F. (2011). Nanoparticle mediated delivery of pure P53 supercoiled plasmid DNA for gene therapy. Journal of Controlled Release, 156(2), 212–222.
Paleček, E., Ostatná, V., Černocká, H., Joerger, A. C., & Fersht, A. R. (2011). Electrocatalytic monitoring of metal binding and mutation-induced conformational changes in p53 at picomole level. Journal of the American Chemical Society, 133(18), 7190–7196.
Will, K., Warnecke, G., Bergmann, S., & Deppert, W. (1995). Species- and tissue-specific expression of the C-terminal alternatively spliced form of the tumor suppressor p53. Nucleic Acids Research, 23(20), 4023–4028.
Miyajima, K., Tamiya, S., Oda, Y., Adachi, T., Konomoto, T., Toyoshiba, H., Masuda, K., & Tsuneyoshi, M. (2001). Relative quantitation of p53 and MDM2 gene expression in leiomyosarcoma; real-time semi-quantitative reverse transcription-polymerase chain reaction. Cancer Letters, 164(2), 177–188.
Narayanaswami, G., & Taylor, P. D. (2002). Site-directed mutagenesis of exon 5 of p53: purification, analysis, and validation of amplicons for DHPLC. Genetic Testing, 6(3), 177–184.
Behn, M., & Schuermann, M. (1998). Sensitive detection of p53 gene mutations by a ‘mutant enriched’ PCR-SSCP technique. Nucleic Acids Research, 26(5), 1356–1358.
Van Orsouw, N. J., Dhanda, R. K., Rines, R. D., Smith, W. M., Sigalas, I., Eng, C., et al. (1998). Rapid design of denaturing gradient-based two-dimensional electrophoretic gene mutational scanning tests. Nucleic Acids Research, 26(10), 2398–2406.
Wang, J., Rivas, G., Cai, X., Chicharro, M., Parrado, C., Dontha, N., Begleiter, A., Mowat, M., Palecek, E., & Nielsen, P. E. (1997). Detection of point mutation in the p53 gene using peptide nucleic acid biosensor. Analytica Chimica Acta, 344(1–2), 111–118.
Noguchi, S., Koyama, H., Kasugai, T., Tsuji, N., Tsuda, H., Akiyama, F., Motomura, K., & Inaji, H. (1998). The possible prognostic significance of p53 immunostaining status of the primary tumor in patients developing local recurrence after breast-conserving surgery. Oncology, 55(5), 450–455.
Barnes, D. M., Dublin, E. A., Fisher, C. J., Levison, D. A., & Millis, R. R. (1993). Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Human Pathology, 24(5), 469–476.
Henke, R. P., Kruger, E., Ayhan, N., Hubner, D., Hammerer, P., & Huland, H. (1994). Immunohistochemical detection of p53 protein in human prostatic cancer. Journal of Urology, 152(4), 1297–1301.
Marquette, C. A., Degiuli, A., Imbert-Laurenceau, E., Mallet, F., Chaix, C., Mandrand, B., et al. (2005). Latex bead immobilisation in PDMS matrix for the detection of p53 gene point mutation and anti-HIV-1 capsid protein antibodies. Analytical and Bioanalytical Chemistry, 381(5), 1019–1024.
Xia, N., Liu, L., Yi, X., & Wang, J. (2009). Studies of interaction of tumor suppressor p53 with apo-MT using surface plasmon resonance. Analytical and Bioanalytical Chemistry, 395(8), 2569–2575.
Jiang, T., Minunni, M., Wilson, P., Zhang, J., Turner, A. P., & Mascini, M. (2005). Detection of TP53 mutation using a portable surface plasmon resonance DNA-based biosensor. Biosensors and Bioelectronics, 20(10), 1939–1945.
Han, S. H., Kim, S. K., Park, K., Yi, S. Y., Park, H. J., Lyu, H. K., Kim, M., & Chung, B. H. (2010). Detection of mutant p53 using field-effect transistor biosensor. Analytica Chimica Acta, 665(1), 79–83.
Chen, C. P., Ganguly, A., Lu, C. Y., Chen, T. Y., Kuo, C. C., Chen, R. S., Tu, W. H., Fischer, W. B., Chen, K. H., & Chen, L. C. (2011). Ultrasensitive in situ label-free DNA detection using a GaN nanowire-based extended-gate field-effect-transistor sensor. Analytical Chemistry, 83(6), 1938–1943.
Domenici, F., Bizzarri, A. R., & Cannistraro, S. (2012). Surface-enhanced Raman scattering detection of wild-type and mutant p53 proteins at very low concentration in human serum. Analytical Biochemistry, 421(1), 9–15.
Marquette, C. A., Lawrence, M. F., & Blum, L. J. (2006). DNA covalent immobilization onto screen-printed electrode networks for direct label-free hybridization detection of p53 sequences. Analytical Chemistry, 78(3), 959–964.
Wang, J., Zhu, X., Tu, Q., Guo, Q., Zarui, C. S., Momand, J., Sun, X. Z., & Zhou, F. (2008). Capture of p53 by electrodes modified with consensus DNA duplexes and amplified voltammetric detection using ferrocene-capped gold nanoparticle/streptavidin conjugates. Analytical Chemistry, 80(3), 769–774.
Rippin, T. M., Freund, S. M. V., Veprintsev, D. B., & Fersht, A. R. (2002). Recognition of DNA by p53 core domain and location of intermolecular contacts of cooperative binding. Journal of Molecular Biology, 319(2), 351–358.
Portefaix, J. M., Fanutti, C., Granier, C., Crapez, E., Perham, R., Grenier, J., Pau, B., & del Rio, M. (2002). Detection of anti-p53 antibodies by ELISA using p53 synthetic or phage-displayed peptides. Journal of Immunological Methods, 259(1–2), 65–75.
Xue, P., Zhang, K., Zhang, Z., Li, Y., Liu, F., Sun, Y., Zhang, X., Song, C., Fu, A., Jin, B., & Yang, K. (2012). Highly sensitive chemiluminescent analysis of residual bovine serum albumin (BSA) based on a pair of specific monoclonal antibodies and peroxyoxalate-glyoxaline-PHPPA dimer chemiluminescent system in vaccines. Applied Biochemistry and Biotechnology, 166(6), 1604–1614.
Kugimiya, A., & Fukada, R. (2015). Chemiluminescence detection of serine, proline, glycine, asparagine, leucine, and histidine by using corresponding aminoacyl-tRNA synthetases as recognition elements. Applied Biochemistry and Biotechnology, 176(4), 1195–1202.
Chuanlai, X., Cifang, P., Kai, H., Zhengyu, J., & Wukang, W. (2006). Chemiluminescence enzyme immunoassay (CLEIA) for the determination of chloramphenicol residues in aquatic tissues. Luminescence, 21(2), 126–128.
Lin, S., Han, S. Q., Liu, Y. B., Xu, W. G., & Guan, G. Y. (2005). Chemiluminescence immunoassay for chloramphenicol. Analytical and Bioanalytical Chemistry, 382(5), 1250–1255.
Xin, T. B., Wang, X., Jin, H., Liang, S. X., Lin, J. M., & Li, Z. J. (2009). Development of magnetic particle-based chemiluminescence enzyme immunoassay for the detection of 17beta-estradiol in environmental water. Applied Biochemistry and Biotechnology, 158(3), 582–594.
Yang, X. Y., Guo, Y. S., Bi, S., & Zhang, S. S. (2009). Ultrasensitive enhanced chemiluminescence enzyme immunoassay for the determination of alpha-fetoprotein amplified by double-codified gold nanoparticles labels. Biosensors and Bioelectronics, 24(8), 2707–2711.
Natrajan, A., & Wen, D. (2011). Facile N-alkylation of acridine esters with 1,3-propane sultone in ionic liquids. Green Chemistry, 13(4), 913.
Pingarrón, J. M., Yáñez-Sedeño, P., & González-Cortés, A. (2008). Gold nanoparticle-based electrochemical biosensors. Electrochimica Acta, 53(19), 5848–5866.
Xu, X., Deng, C., Gao, M., Yu, W., Yang, P., & Zhang, X. (2006). Synthesis of magnetic microspheres with immobilized metal ions for enrichment and direct determination of phosphopeptides by matrix-assisted laser desorption ionization mass spectrometry. Advanced Materials, 18(24), 3289–3293.
Deng, Y., Qi, D., Deng, C., Zhang, X., & Zhao, D. (2008). Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. Journal of the American Chemical Society, 130(1), 28–29.
Schlaeppi, J.-M. A., Kessler, A., & Foery, W. (1994). Development of a magnetic particle-based automated chemiluminescent immunoassay for triasulfuron. Journal of Agricultural and Food Chemistry, 42(9), 1914–1919.
Malar, C. G., Seenuvasan, M., & Kumar, K. S. (2018). Prominent study on surface properties and diffusion coefficient of urease-conjugated magnetite nanoparticles. Applied Biochemistry and Biotechnology.
Pudlarz, A. M., Czechowska, E., Ranoszek-Soliwoda, K., Tomaszewska, E., Celichowski, G., Grobelny, J., & Szemraj, J. (2018). Immobilization of recombinant human catalase on gold and silver nanoparticles. Applied Biochemistry and Biotechnology.
Wang, X., Wang, X., Wang, X., Chen, F., Zhu, K., Xu, Q., & Tang, M. (2013). Novel electrochemical biosensor based on functional composite nanofibers for sensitive detection of p53 tumor suppressor gene. Analytica Chimica Acta, 765, 63–69.
Raoof, J. B., Ojani, R., Golabi, S. M., Hamidi-Asl, E., & Hejazi, M. S. (2011). Preparation of an electrochemical PNA biosensor for detection of target DNA sequence and single nucleotide mutation on p53 tumor suppressor gene corresponding oligonucleotide. Sensors and Actuators B: Chemical, 157(1), 195–201.
Chen, X., He, C., Zhang, Z., & Wang, J. (2013). Sensitive chemiluminescence detection of wild-type p53 protein captured by surface-confined consensus DNA duplexes. Biosensors and Bioelectronics, 47, 335–339.
Luo, X.-W., Du, F.-J., Wu, Y., Gao, L.-J., & Li, X.-X. (2013). Electrochemical DNA sensor for determination of p53 tumor suppressor gene incorporating gold nanoparticles modification. Chinese Journal of Analytical Chemistry, 41(11), 1664–1668.
Afsharan, H., Navaeipour, F., Khalilzadeh, B., Tajalli, H., Mollabashi, M., Ahar, M. J., & Rashidi, M. R. (2016). Highly sensitive electrochemiluminescence detection of p53 protein using functionalized Ru-silica nanoporous@gold nanocomposite. Biosensors and Bioelectronics, 80, 146–153.
Tiwari, A., Deshpande, S. R., Kobayashi, H., & Turner, A. P. F. (2012). Detection of p53 gene point mutation using sequence-specific molecularly imprinted PoPD electrode. Biosensors and Bioelectronics, 35(1), 224–229.
Qi, Y., Xiu, F. R., Zheng, M., & Li, B. (2016). A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: sensitivity, selectivity and mechanism. Biosensors and Bioelectronics, 83, 243–249.
Esteban-Fernandez de Avila, B., Araque, E., Campuzano, S., Pedrero, M., Dalkiran, B., Barderas, R., et al. (2015). Dual functional graphene derivative-based electrochemical platforms for detection of the TP53 gene with single nucleotide polymorphism selectivity in biological samples. Analytical Chemistry, 87(4), 2290–2298.
Altintas, Z., & Tothill, I. E. (2012). DNA-based biosensor platforms for the detection of TP53 mutation. Sensors and Actuators, B: Chemical, 169, 188–194.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 81371642) and the 111 Project (B14040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
ESM 1
(DOCX 5558 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Yao, M., Fang, X. et al. Novel Competitive Chemiluminescence DNA Assay Based on Fe3O4@SiO2@Au-Functionalized Magnetic Nanoparticles for Sensitive Detection of p53 Tumor Suppressor Gene. Appl Biochem Biotechnol 187, 152–162 (2019). https://doi.org/10.1007/s12010-018-2808-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2808-1