Abstract
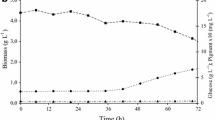
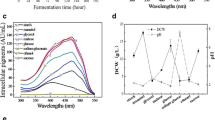
To reduce environmental problems caused by glycerine accumulation and to make the production of biodiesel more profitable, crude glycerin without treatment was used as substrate for obtaining higher value-added bioproducts. Monascus ruber is a filamentous fungus that produces pigments, particularly red ones, which are used for coloring foods (rice wine and meat products). The interest in developing pigments from natural sources is increasing due to the restriction of using synthetic dyes. The effects of temperature, pH, microorganism morphology, aeration, nitrogen source, and substrates have been studied in the cultivation of M. ruber. In this work, it was observed that light intensity is also an important factor that should be considered for understanding the metabolism of the fungus. In M. ruber cultivation, inhibition of growth and pigment production was observed in Petri dishes and blaffed flasks exposed to direct illumination. Growth and pigment production were higher in Petri dishes and flasks exposed to red light and in the absence of light. Radial growth rate of M. ruber in plates in darkness was 1.50 mm day−1 and in plates exposed to direct illumination was 0.59 mm day−1. Maximum production of red pigments (8.32 UA) and biomass (8.82 g L−1) were obtained in baffled flasks covered with red film and 7.17 UA of red pigments, and 7.40 g L−1 of biomass was obtained in flasks incubated in darkness. Under conditions of 1248 lux of luminance, the maximum pigment production was 4.48 UA, with production of 6.94 g L−1 of biomass, indicating that the fungus has photoreceptors which influence the physiological responses.




Similar content being viewed by others
References
Wong, H. C., & Koehler, P. (1981). Production and isolation of an antibiotic from Monascus purpureus and its relationship to pigment production. Journal of Food Science, 46, 589–592.
Domínguez-espinosa, R. M., & Webb, C. (2003). Submerged fermentation in wheat substrates for production of Monascus pigments. World Journal of Microbiology and Biotechnology, 19, 329–336.
Pastrana, L., Blanc, P. J., Santerre, A. L., Loret, M. O., & Goma, G. (1995). Production of red pigments by Monascus ruber in synthetic media with a strictly controlled nitrogen source. Process Biochemistry, 30, 333–341.
Hamdi, M., Blanc, P. J., & Goma, G. (1996). Effect of aeration conditions on the production of red pigments by Monascus purpureus growth on prickly pear juice. Process Biochemistry, 31, 543–547.
Yongsmith, B., Tabloka, W., Yongmanitchai, W., & Bavavoda, R. (1993). Culture conditions for yellow pigment formation by Monascus sp. KB 10 grown on cassava medium. World Journal of Microbiology and Biotechnology, 9, 85–90.
Babitha, S., Soccol, C. R., & Pandey, A. (2006). Jackfruit seed – a novel substrate for the production of Monascus pigments throught solid-state fermentation. Food Technology and Biotechnology, 44, 465–471.
Mukherjee, G., & Singh, S. K. (2011). Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochemistry, 46, 188–192.
Jung, H., Kim, C., Kim, K., & Shin, C. S. (2003). Color characteristics of Monascus pigments derived by fermentation with various amino acids. Journal of Agricultural and Food Chemistry, 5, 1302–1306.
Ahn, J., Jung, J., Hyung, W., Haam, H., & Shin, C. (2006). Enhancement of Monascus pigment production by the culture of Monascus spp. J101 at low temperature. Biotechnology Progress, 22, 338–340.
Vendruscolo, F., Pitol, L. O., Carciofi, B. A. M., Moritz, D. E., Laurindo, J. B., Schmidell, W., & Ninow, J. L. (2010). Construction and application a vane system in a rotational rheometer for determination of the rheological properties of Monascus ruber CCT 3802. Journal of Biorheology, 24, 29–35.
Carels, M., & Shepherd, D. (1975). Sexual reproductive cycle of Monascus in submerged shaken culture. Journal of Bacteriology, 122, 288–294.
Miyake, T., Mori, A., Okuno, A. K. T., Usui, Y., Sammoto, F. S. H., & Kariyama, A. W. M. (2005). Light effects on cell development and secondary metabolism in Monascus. Journal of Industrial Microbiology and Biotechnology, 32, 103–108.
Zheng, W., Zhang, M., Zhao, Y., Miao, K., & Jiang, H. (2009). NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Bioresource Technology, 100, 4481–4487.
Velmurugan, P., Lee, Y. H., Venil, C. K., Lakshmanaperumalsamy, P., Chae, J. C., & Oh, B. T. (2010). Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. Journal of Bioscience and Bioengineering, 109, 346–350.
Hajjaj, H., Blanc, P. J., Groussac, E., Uribelarrea, J. L., Goma, G., & Loubiere, L. (2000). Kinetics analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enzyme and Microbial Technology, 27, 619–625.
Hamdi, M., Blanc, P. J., Loret, M. O., & Goma, J. L. (1997). A new process for red pigment production by submerged culture of Monascus purpureus. Bioprocess Engineering, 17, 75–79.
Tseng, Y. Y., Chen, M. T., & Lin, C. F. (2000). Growth, pigment production and protease activity of Monascus purpureusas affected by salt, sodium nitrite, polyphosphate and various sugars. Journal of Applied Microbiology, 88, 31–37.
Hamano, P. S., & Kilikian, B. V. (2006). Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Brazilian Journal of Chemical Engineering, 23, 443–449.
Silveira, S. T., Daroit, D. J., & Brandelli, A. (2008). Pigment production by Monascus purpureus in grape waste using factorial design. LWT - Food Science and Technology, 41, 170–174.
Xu, W. (2001). Study on the liquid fermentation to produce Monascus pigment with corn starch and antibacteria. Advanced Materials Research, 1336, 183–185.
Kongruang, S. (2011). Growth kinetics of biopigment production by Thai isolated Monascus purpureus in a stirred tank bioreactor. Journal of Industrial Microbiology and Biotechnology, 38, 93–99.
Meinicke, R. M., Vendruscolo, F., Moritz, D. E., Oliveira, D., Schmidell, W., Samohyl, R. W., & Ninow, J. L. (2012). Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatalysis and Agricultural Biotechnology, 3, 238–242.
Gomes, M. C. S., Arroyo, P. A., & Pereira, N. C. P. (2011). Biodiesel production from degummed soybean oil and glycerol removal using ceramic membrane. Journal of Membrane Science, 378, 453–461.
Leung, D. Y. C., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87, 1083–1095.
Leoneti, A. B., Aragão-Leoneti, V., & Oliveira, S. V. W. B. (2012). Glycerol as a by-product of biodiesel production in Brazil: alternatives for the use of unrefined glycerol. Renewable Energy, 45, 138–145.
Hajjaj, H., Blanc, P. J., Groussac, E., Goma, G., Uribelarrea, J. L., & Loubiere, P. (1999). Improvement of red pigment/citrinin production ratio as a function of environmental conditions by Monascus ruber. Biotechnology and Bioengineering, 64, 497–501.
Babitha, S., Carvalho, J. C., Soccol, C. R., & Pandey, A. (2008). Effect of light on growth, pigment production and culture morphology of Monascus purpureus in solid-state fermentation. World Journal of Microbiology and Biotechnology, 24, 2671–2675.
Gabiatti Junior, C., Vendruscolo, F., Piaia, J. C. Z., Rodrigues, R. C., Durrant, L. R., & Costa, J. A. V. (2004). Radial growth rate as a tool for the selection of filamentous fungi for use in bioremediation. Brazilian Archives of Biology and Technology, 47, 225–232.
Hamano, P. S., Orozco, S. F. V. B., & Kilikian, B. V. (2005). Concentration determination of extracellular and intracellular red pigments produced by Monascus spp. Brazilian Archives of Biology and Technology, 48, 43–49.
Juzlova, P., Martinkova, L., & Kren, V. (1996). Secondary metabolities of the fungus Monascus: a review. Journal of Industrial Microbiology, 16, 163–170.
Kim, H. J., Kim, J. H., Oh, H. J., & Shin, C. S. (2002). Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochemistry, 38, 649–655.
Thomson, J., & He, B. (2006). Characterization of crude glycerol from biodiesel production from multiple feedstocks. Applied Engineering in Agriculture, 22, 261–265.
Schmidt-Heydt, M., Rüfer, C., Raupp, F., Bruchmann, A., Perrone, G., & Geisen, R. (2011). Influence of light on food relevant fungi with emphasis on ochratoxin producing species. International Journal of Food Microbiology, 145, 229–237.
Carvalho, J. C., Oishi, B. O., Pandey, A., & Soccol, C. R. (2005). Biopigments from Monascus: strain selection, citrinin production and color stability. Brazilian Archives of Biology and Technology, 48, 885–894.
Lee, B. K., Park, N. H., Piao, H. Y., & Chung, W. J. (2001). Production of red pigments by Monascus purpureus in submerged culture. Biotechnology and Bioprocess Engineering, 6, 341–346.
Danesi, E. D. G., Rangel-Yagui, C. O., Carvalho, J. C. M., & Sato, S. (2004). Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass and Bioenergy, 26, 329–335.
Ramirez, D. A., Munoz, S. V., Atehortua, L., & Michel, F. C., Jr. (2010). Effects of different wavelengths of light on lignin peroxidase production by the white-rot fungi Phanerochaete chrysosporium grown in submerged cultures. Bioresource Technology, 101, 9213–9220.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bühler, R.M.M., Müller, B.L., Moritz, D.E. et al. Influence of Light Intensity on Growth and Pigment Production by Monascus ruber in Submerged Fermentation. Appl Biochem Biotechnol 176, 1277–1289 (2015). https://doi.org/10.1007/s12010-015-1645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1645-8