Abstract
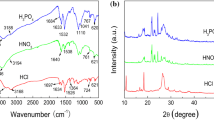
A series of Zn2+ based hydrophobic coordination polymers (CPs) have been synthesized by varying methylene spacers length (m = 1 to m = 6) of aliphatic dicarboxylic acids followed by reaction with the zinc acetate by using hydrothermal method. The synthesized CPs were coated on the mild steel (MS) specimens to investigate the effect of methylene spacers length of dicarboxylic acids on hydrophobicity and corrosion behavior. The synthesized CPs and their coatings on MS have been characterized by various instrumental techniques such as Fourier transform infrared (FTIR) spectroscopy, powder X-ray diffraction (PXRD), Field Emission Scanning Electron Microscopy (FE-SEM), water contact angle (WCA) measurements. FTIR and PXRD measurements confirm the formation of CPs whereas the FE-SEM images of suberic acid polymer (SUAP) reveal compact distribution of sphere-like aggregates. WCA measurements showed that the bare MS including CPs coatings on MS up to methylene spacers length m = 4 is hydrophilic in nature; however, the coating of longest methylene spacers length CP (SUAP, m = 6) showed hydrophobic nature with water contact angle of ~ 108.6°. Furthermore, the obtained hydrophobic SUAP CP with polyvinylidene fluoride (PVDF) as binder was coated on MS to study its anticorrosion behavior for the protection of MS. The anticorrosion performance of the developed SUAP coating was investigated by open-circuit potential (OCP) and potentiodynamic polarization techniques. The corrosion rate has been calculated from potentiodynamic polarization curves for the bare MS and SUAP coated MS showed significant improvement in the anticorrosion behavior of the developed SUAP coating.









Similar content being viewed by others
References
Hansson, CM, “The Impact of Corrosion on Society.” A Phys. Metall. Mater. Sci., 42 (10) 2952–2962. https://doi.org/10.1007/s11661-011-0703-2 (2011)
Zhang, Y, DesRoches, R, Tien, I, “Impact of Corrosion on Risk Assessment of Shear-Critical and Short Lap-Spliced Bridges.” Eng. Struct., 189 260–271. https://doi.org/10.1016/j.engstruct.2019.03.050 (2019)
Chaouiki, A, Lgaz, H, Salghi, R, Chafiq, M, Oudda, H, Bhat, KS, Cretescu, I, Ali, IH, Marzouki, R, Chung, IM, “Assessing the Impact of Electron-Donating-Substituted Chalcones on Inhibition of Mild Steel Corrosion in HCl Solution: Experimental Results and Molecular-Level Insights.” Colloids Surf. A Physicochem. Eng. Asp., 588 512–519. https://doi.org/10.1016/j.colsurfa.2019.124366 (2020)
Suleiman, RK, Kumar, AM, Adesina, AY, Al-Badour, FA, Meliani, MH, Saleh, TA, “Hybrid Organosilicon-Metal Oxide Composites and Their Corrosion Protection Performance for Mild Steel in 3.5% NaCl Solution.” Corros. Sci., 169 108637–108658. https://doi.org/10.1016/j.corsci.2020.108637 (2020)
Saleh, MM, Mahmoud, MG, Abd El-Lateef, HM, “Comparative Study of Synergistic Inhibition of Mild Steel and Pure Iron by 1-Hexadecylpyridinium Chloride and Bromide Ions.” Corros. Sci., 154 70–79. https://doi.org/10.1016/j.corsci.2019.03.048 (2019)
Khodair, ZT, Khadom, AA, Jasim, HA, “Corrosion Protection of Mild Steel in Different Aqueous Media via Epoxy/Nanomaterial Coating: Preparation, Characterization and Mathematical Views.” J. Mater. Res. Technol., 8 (1) 424–435. https://doi.org/10.1016/j.jmrt.2018.03.003 (2019)
Zhang, W, Wang, Y, Li, HJ, Liu, Y, Tao, R, Guan, S, Li, Y, Wu, YC, “Synergistic Inhibition Effect of 9-(4-Chlorophenyl)-1,2,3,4-Tetrahydroacridines and Tween-80 for Mild Steel Corrosion in Acid Medium.” J. Phys. Chem. C, 123 (23) 14480–14489. https://doi.org/10.1021/acs.jpcc.9b02595 (2019)
Nene, SS, Frank, M, Liu, K, Sinha, S, Mishra, RS, McWilliams, BA, Cho, KC, “Corrosion-Resistant High Entropy Alloy with High Strength and Ductility.” Scr. Mater., 166 168–172. https://doi.org/10.1016/j.scriptamat.2019.03.028 (2019)
Xu, W, Wei, L, Zhang, Z, Liu, Y, Chou, KC, Fan, H, Li, Q, “Effects of Lanthanum Addition on the Microstructure and Corrosion Resistance of Galvanized Coating.” J. Alloys Compd., 784 859–868. https://doi.org/10.1016/j.jallcom.2019.01.075 (2019)
Wu, H, Zhang, L, Liu, C, Mai, Y, Zhang, Y, Jie, X, “Deposition of Zn-G/Al Composite Coating with Excellent Cathodic Protection on Low-Carbon Steel by Low-Pressure Cold Spraying.” J. Alloys Compd., 821 153483–153495. https://doi.org/10.1016/j.jallcom.2019.153483 (2020)
Farooq, A, Hamza, M, Ahmed, Q, Deen, KM, “Evaluating the Performance of Zinc and Aluminum Sacrificial Anodes in Artificial Seawater.” Electrochim. Acta, 314 135–141. https://doi.org/10.1016/j.electacta.2019.05.067 (2019)
Chen, C, He, Y, Xiao, G, Zhong, F, Li, H, Wu, Y, Chen, J, “Synergistic Effect of Graphene Oxide@Phosphate-Intercalated Hydrotalcite for Improved Anti-Corrosion and Self-Healable Protection of Waterborne Epoxy Coating in Salt Environments.” J. Mater. Chem. C, 7 (8) 2318–2326. https://doi.org/10.1039/c8tc06487c (2019)
Nazeer, AA, Madkour, M, “Potential Use of Smart Coatings for Corrosion Protection of Metals and Alloys: A Review.” J. Mol. Liq., 253 11–22. https://doi.org/10.1016/j.molliq.2018.01.027 (2018)
Huang, TC, Lai, GH, Li, CE, Tsai, MH, Wan, PY, Chung, YH, Lin, MH, “Advanced Anti-Corrosion Coatings Prepared from α-Zirconium Phosphate/Polyurethane Nanocomposites.” RSC Adv., 7 (16) 9908–9913. https://doi.org/10.1039/c6ra27588e (2017)
Lu, X, Liu, Y, Zhou, C, Zhang, W, Xin, Z, “Corrosion Protection of Hydrophobic Bisphenol A-Based Polybenzoxazine Coatings on Mild Steel.” RSC Adv., 6 (7) 5805–5811. https://doi.org/10.1039/c5ra22980d (2016)
Sørensen, PA, Kiil, S, Dam-Johansen, K, Weinell, CE, “Anticorrosive Coatings: A Review.” J. Coat. Technol. Res., 6 (2) 135–176. https://doi.org/10.1007/s11998-008-9144-2 (2009)
Vazirinasab, E, Jafari, R, Momen, G, “Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review.” Surf. Coat. Technol., 341 40–56. https://doi.org/10.1016/j.surfcoat.2017.11.053 (2018)
Song, JW, Ma, MC, Fan, LW, “Understanding the Temperature Dependence of Contact Angles of Water on a Smooth Hydrophobic Surface under Pressurized Conditions: An Experimental Study.” Langmuir, 36 (32) 9586–9595. https://doi.org/10.1021/acs.langmuir.0c01671 (2020)
Furtat, P, Lenz-Leite, M, Ionescu, E, MacHado, RAF, Motz, G, “Synthesis of Fluorine-Modified Polysilazanes: Via Si-H Bond Activation and Their Application as Protective Hydrophobic Coatings.” J. Mater. Chem. A, 5 (48) 25509–25521. https://doi.org/10.1039/c7ta07687h (2017)
Bakshi, MI, Ahmad, S, “In-Situ Synthesis of Synergistically Active Ceria Doped Polypyrrole Oleo-Polyesteramide Hybrid Nanocomposite Coatings: Corrosion Protection and Flame Retardancy Behaviour.” Prog. Org. Coat., 147 105778–105795. https://doi.org/10.1016/j.porgcoat.2020.105778 (2020)
Sharma, S, Kumar, A, “Recent Advances in Metallic Corrosion Inhibition: A Review.” J. Mol. Liq., 322 114862–114886. https://doi.org/10.1016/j.molliq.2020.114862 (2020)
Dixit, MK, Kumar, Y, Shukla, J, Chinthakuntla, M, Dubey, M, “Bis(Acylhydrazone)-Based Bolaamphiphiles: Effect of Spacer Length on Metalloorganogel Formation, Fluorescence and Conductance Properties.” ChemPlusChem, 87 e201900589. https://doi.org/10.1002/cplu.201900589 (2022)
Pandey, VK, Dixit, MK, Manneville, S, Bucher, C, Dubey, M, “A Multi-Stimuli Responsive Conductive Sonometallogel: A Mechanistic Insight into the Role of Ultrasound in Gelation.” J. Mater. Chem. A, 5 (13) 6211–6218. https://doi.org/10.1039/c7ta00854f (2017)
Wu, LYL, Soutar, AM, Zeng, XT, “Increasing Hydrophobicity of Sol-Gel Hard Coatings by Chemical and Morphological Modifications.” Surf. Coat. Technol., 198 420–424. https://doi.org/10.1016/j.surfcoat.2004.10.050 (2004)
Lv, D, Shao, H, Gao, X, Lu, K, Lu, H, Ma, H, “Fabrication and Corrosion Resistance Properties of Super-Hydrophobic Coatings on Iron and Steel Substrates by Creating Micro-/Nano-Structures and Modifying Rough Surfaces.” RSC Adv., 6 (96) 93419–93427. https://doi.org/10.1039/c6ra17655k (2016)
Song, JH, Kim, Y, Lim, KS, Kang, DW, Lee, WR, Hong, CS, “Phase Transformation, Exceptional Quenching Efficiency, and Discriminative Recognition of Nitroaromatic Analytes in Hydrophobic, Nonporous Zn(II) Coordination Frameworks.” Inorg. Chem., 56 (1) 305–312. https://doi.org/10.1021/acs.inorgchem.6b02178 (2017)
Dubey, M, Kumar, A, Pandey, DS, “Homochiral Coordination Polymeric Gel: Zn2+-Induced Conformational Changes Leading to J-Aggregated Helical Fibres Formation.” Chem. Commun., 50 (14) 1675–1677. https://doi.org/10.1039/c3cc47359g (2014)
Alibakhshi, E, Akbarian, M, Ramezanzadeh, M, Ramezanzadeh, B, Mahdavian, M, “Evaluation of the Corrosion Protection Performance of Mild Steel Coated with Hybrid Sol-Gel Silane Coating in 3.5 Wt.% NaCl Solution.” Prog. Org. Coat., 123 190–200. https://doi.org/10.1016/j.porgcoat.2018.07.008 (2018)
Choudhary, S, Garg, A, Mondal, K, “Relation Between Open Circuit Potential and Polarization Resistance with Rust and Corrosion Monitoring of Mild Steel.” J. Mater. Eng. Perform., 25 (7) 2969–2976. https://doi.org/10.1007/s11665-016-2112-6 (2016)
Verma, C, Olasunkanmi, LO, Bahadur, I, Lgaz, H, Quraishi, MA, Haque, J, Sherif, ESM, Ebenso, EE, “Experimental, Density Functional Theory and Molecular Dynamics Supported Adsorption Behavior of Environmental Benign Imidazolium Based Ionic Liquids on Mild Steel Surface in Acidic Medium.” J. Mol. Liq., 273 1–15. https://doi.org/10.1016/j.molliq.2018.09.139 (2019)
Dagdag, O, Safi, Z, Wazzan, N, Erramli, H, Guo, L, Mkadmh, AM, Verma, C, Ebenso, EE, El Gana, L, El Harfi, A, “Highly Functionalized Epoxy Macromolecule as an Anti-Corrosive Material for Carbon Steel: Computational (DFT, MDS), Surface (SEM-EDS) and Electrochemical (OCP, PDP, EIS) Studies.” J. Mol. Liq., 302 10–25. https://doi.org/10.1016/j.molliq.2020.112535 (2020)
Caldona, EB, de Leon, ACC, Pajarito, BB, Advincula, RC, “Novel Anti-Corrosion Coatings from Rubber-Modified Polybenzoxazine-Based Polyaniline Composites.” Appl. Surf. Sci., 422 162–171. https://doi.org/10.1016/j.apsusc.2017.05.083 (2017)
Stephens, LI, Perry, SC, Gateman, SM, Lacasse, R, Schulz, R, Mauzeroll, J, “Development of a Model for Experimental Data Treatment of Diffusion and Activation Limited Polarization Curves for Magnesium and Steel Alloys.” J. Electrochem. Soc., 164 (11) 3576–3582. https://doi.org/10.1149/2.0591711jes (2017)
Hamidon, TS, Hussin, MH, “Susceptibility of Hybrid Sol-Gel (TEOS-APTES) Doped with Caffeine as Potent Corrosion Protective Coatings for Mild Steel in 3.5 Wt.% NaCl.” Prog. Org. Coat., 140 105478–105494. https://doi.org/10.1016/j.porgcoat.2019.105478 (2020)
Kahyarian, A, Schumaker, A, Brown, B, Nesic, S, “Acidic Corrosion of Mild Steel in the Presence of Acetic Acid: Mechanism and Prediction.” Electrochim. Acta, 258 639–652. https://doi.org/10.1016/j.electacta.2017.11.109 (2017)
Liu, RL, Scully, JR, Williams, G, Birbilis, N, “Reducing the Corrosion Rate of Magnesium via Microalloying Additions of Group 14 and 15 Elements.” Electrochim. Acta, 260 184–195. https://doi.org/10.1016/j.electacta.2017.11.062 (2018)
Acknowledgments
M. Dubey acknowledges the Department of Science and Technology, New Delhi, India, for financial support under a DST-INSPIRE Faculty award (IFA-14/CH-156). YK and CM thank IIT Indore for fellowship and UGC New Delhi for Senior Research Fellowship, respectively. AK extend his appreciation to the Deanship of Scientific Research at King Khalid University for contribution through research groups program under grant number RGP2/188/43. We are grateful to SIC IIT Indore for extending instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, Y., Pandey, S., Yashpal, S. et al. Corrosion protection ability of hydrophobic zinc based coordination polymers on mild steel surface. J Coat Technol Res 20, 1145–1155 (2023). https://doi.org/10.1007/s11998-022-00734-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00734-7