Abstract
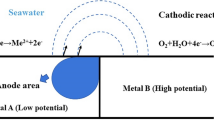
In recent years, many breakthroughs in research on graphene as a corrosion-resistant coating for metal have been witnessed due to its excellent properties like chemical stability, mechanical strength, permeability, etc. The present review discusses graphene and its composite as an anticorrosive coating for marine applications. The economic and environmental losses caused by the corrosion in day-to-day life are large, and coatings are one of the preventive measures to reduce these losses. The corrosion gets accelerated when some significant factors like salinity, pH, water velocity, temperature, and dissolved oxygen content also come into picture. The excellent physical and chemical properties for graphene like high strength electrical conductivity, large surface area, etc., make it a potential candidate for a number of applications. The different techniques for the synthesis of graphene and its derivatives like graphene oxide and reduced graphene oxide have been also briefed in this review. The pure graphene coatings have shown excellent anticorrosive properties. However, defects in pure graphene coating have a substantial impact compared to multilayering for anticorrosive behavior. Graphene-based composites are more durable and reliable than pure graphene coating. The various techniques for the synthesis of graphene-based polymer coatings are solution mixing, melt mixing, and in situ polymerization. In the marine environment, graphene should be dispersed uniformly parallel to the metallic surface to provide excellent erosion corrosion properties. However, cathodic protection is a crucial issue in organic coatings, and zinc-rich graphene coatings are effective in case of defects in the coating. Other functional properties of graphene-based polymeric coatings like antifouling, hydrophobicity, better mechanical and wear properties, UV protection, and electrical conductivity give an edge to these coatings in marine conditions.

(Source: Scopus, accessed 20 October 2022)








Similar content being viewed by others
References
Shifler, DA, “Understanding Material Interactions in Marine Environments to Promote Extended Structural Life.” Corros. Sci., 47 (10) 2335–2352. https://doi.org/10.1016/j.corsci.2004.09.027 (2005)
Bowman, E, et al. “International Measures of Prevention, Application, and Economics of Corrosion Technologies Study.” NACE Int., 216 2–3 (2016)
Selim, MS, et al. “Recent Progress in Marine Foul-release Polymeric Nanocomposite Coatings.” Prog. Mater. Sci., 87 1–32. https://doi.org/10.1016/j.pmatsci.2017.02.001 (2017)
Bhoj, Y, Tharmavaram, M, Rawtani, D, “A Comprehensive Approach to Antifouling Strategies in Desalination, Marine Environment, and Wastewater Treatment.” Chem. Phys. Impact, 2 108. https://doi.org/10.1016/j.chphi.2020.100008 (2021)
Yang, WJ, Neoh, KG, Kang, ET, Teo, SLM, Rittschof, D, “Polymer Brush Coatings for Combating Marine Biofouling.” Prog. Polym. Sci., 39 (5) 1017–1042. https://doi.org/10.1016/j.progpolymsci.2014.02.002 (2014)
Pedeferri, P, “General Principles of Corrosion.” In: General Principles of Corrosion, p. 4. (2018)
Chandler, KA, “Marine Environment.” In: Marine and Offshore Corrosion, pp. 38–49 (1985)
Sørensen, PA, Kiil, S, Dam-Johansen, K, Weinell, CE, “Anticorrosive Coatings: A Review.” J. Coat. Technol. Res., 6 (2) 135–176. https://doi.org/10.1007/s11998-008-9144-2 (2009)
Radhamani, AV, Lau, HC, Ramakrishna, S, “Nanocomposite Coatings on Steel for Enhancing the Corrosion Resistance: A Review.” J. Compos. Mater., 54 (5) 681–701. https://doi.org/10.1177/0021998319857807 (2020)
Wang, X, Li, J, Zhang, J, Sun, Z, Yu, L, “Polyaniline as Marine Antifouling and Corrosion-Prevention Agent.” Synth. Met., 102 1377–1380 (1999)
Sharma, N, Sharma, S, “Anticorrosive Coating of Polymer Composites: A Review.” Mater. Today Proc., 44 4498–4502. https://doi.org/10.1016/j.matpr.2020.10.726 (2020)
Allen, MJ, Tung, VC, Kaner, RB, “Honeycomb Carbon: A Review of Graphene.” Chem. Rev., 110 132–145 (2010)
Geim, AK, Novoselov, KS, “The Rise of Graphene.” Nat. Mater., 6 183–191 (2007)
Georgakilas, V, Perman, JA, Tucek, J, Zboril, R, “Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures.” Chem. Rev.,. https://doi.org/10.1021/cr500304f (2014)
Cui, G, Bi, Z, Zhang, R, Liu, J, Yu, X, Li, Z, “A Comprehensive Review on Graphene-Based Anti-Corrosive Coatings.” Chem. Eng. J., 373 104–121. https://doi.org/10.1016/j.cej.2019.05.034 (2019)
Abd El-Lateef, HM, Abbasov, VM, Aliyeva, LI, Ismayilov, TA, “Corrosion Protection of Steel Pipelines Against CO2 Corrosion-A Review.” Chem. J., 2 (2) 52–63 (2012)
Zheng, Y, Brown, B, Nešić, S, “Electrochemical Study and Modeling of H2S Corrosion of Mild Steel.” Corrosion, 70 (4) 351–365. https://doi.org/10.5006/0937 (2014)
Sun, C, et al. “Synergistic Effect of O2, H2S and SO2 Impurities on the Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 System.” Corros. Sci., 107 193–203. https://doi.org/10.1016/j.corsci.2016.02.032 (2016)
Zou, Y, Wang, J, Zheng, YY, “Electrochemical Techniques for Determining Corrosion Rate of Rusted Steel in Seawater.” Corros. Sci., 53 (1) 208–216. https://doi.org/10.1016/j.corsci.2010.09.011 (2011)
Wen, G, Bai, P, Tian, Y, “A Review of Graphene-Based Materials for Marine Corrosion Protection.” J. Bio-Tribo-Corros., https://doi.org/10.1007/s40735-020-00456-6 (2021)
Funke, W, “Organic Coatings in Corrosion Protection.” Surf. Coat., 2 107–135. https://doi.org/10.1007/978-94-009-1351-6_4 (1988)
Sahu, SC, et al. “A Facile Electrochemical Approach for Development of Highly Corrosion Protective Coatings Using Graphene Nanosheets.” Electrochem. Commun., 32 22–26. https://doi.org/10.1016/j.elecom.2013.03.032 (2013)
Adler, TA, Aylor, D, Bray, A, “Effects of Metallurgical Variables on the Corrosion of Stainless Steels.” Corros. Petrochem. Ind., https://doi.org/10.31399/asm.tb.cpi2.t55030062 (2020)
Cai, YK, Zhao, Y, Zhang, ZK, Ma, XB, Cheng, B, Atmospheric and Marine Corrosion: Influential Environmental Factors and Models. (2018)
Melchers, RE, “Effect on Marine Immersion Corrosion of Carbon Content of Low Alloy Steels.” Corros. Sci., 45 (11) 2609–2625. https://doi.org/10.1016/S0010-938X(03)00068-4 (2003)
Turner, MED, “Corrosion Engineering and Corrosion Science.” Mater. Perform., 19 (10) 51–52. https://doi.org/10.5006/0010-9312-19.6.199 (1980)
Chaves, IA, Melchers, RE, “Failure Prediction of Mild-Steel Welds Due to Climate Change Influenced Marine Corrosion.” The 28th International Ocean and Polar Engineering Conference. (2018)
Melchers, RE, “Mathematical Modelling of the Diffusion Controlled Phase in Marine Immersion Corrosion of Mild Steel.” Corros. Sci., 45 (5) 923–940. https://doi.org/10.1016/S0010-938X(02)00208-1 (2003)
Novoselov, KS, et al. “Electric Field in Atomically Thin Carbon Films.” Science, 306 (5696) 666–669. https://doi.org/10.1126/science.1102896 (2004)
Frank, IW, Tanenbaum, DM, “Mechanical Properties of Suspended Graphene Sheets.” J. Vac. Sci. Technol., 25 2558–2561. https://doi.org/10.1116/1.2789446 (2007)
Min, K, Aluru, NR, Min, K, Aluru, NR, “Mechanical Properties of Graphene Under Shear Deformation.” Appl. Phys. Lett., 98 013113. https://doi.org/10.1063/1.3534787 (2011)
Lee, C, Wei, X, Kysar, JW, Hone, J, “Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene.” Science, 321 385–388. https://doi.org/10.1126/science.1157996 (2008)
Balandin, AA, “Thermal Properties of Graphene and Nanostructured Carbon Materials.” Nat. Mater., 10 (8) 569–581. https://doi.org/10.1038/nmat3064 (2011)
Bolotin, KI, et al. “Ultrahigh Electron Mobility in Suspended Graphene.” Solid State Commun., 146 (9–10) 351–355. https://doi.org/10.1016/j.ssc.2008.02.024 (2008)
Geim, AK, “Nobel Lecture: Random Walk to Graphene.” Rev. Modern Phys., 83 851–862. https://doi.org/10.1103/RevModPhys.83.851 (2011)
Li, X, et al. “Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils.” Science, 324 1312–1314. https://doi.org/10.1126/science.1171245 (2009)
Stankovich, S, et al. “Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide.” Carbon N. Y., 45 (7) 1558–1565. https://doi.org/10.1016/j.carbon.2007.02.034 (2007)
Wang, G, et al., “JPC;C081128192-Synthesis-Characterization-GNsheets.pdf,” pp. 8192–8195, (2008)
Chen, W, Yan, L, Bangal, PR, “JPC1011419885-NaHSO3-GO.pdf,” pp. 19885–19890, (2010)
Chua, CK, Pumera, M, “Chemical Reduction of Graphene Oxide: A Synthetic Chemistry Viewpoint.” Chem. Soc. Rev., 43 (1) 291–312. https://doi.org/10.1039/c3cs60303b (2014)
Tour, JM, “Top-Down Versus Bottom-Up Fabrication of Graphene-Based Electronics.” Chem. Mater., 26 (1) 163–171. https://doi.org/10.1021/cm402179h (2014)
Zhu, Y, et al. “Graphene and Graphene Oxide: Synthesis, Properties, and Applications.” Adv. Mater., 22 (35) 3906–3924. https://doi.org/10.1002/adma.201001068 (2010)
Hummers, WS, Offeman, RE, “Preparation of Graphitic Oxide.” J. Am. Chem. Soc., 208 1937 (1957)
Teijido, R, Ruiz-Rubio, L, Echaide, AG, Vilas-Vilela, JL, Lanceros-Mendez, S, Zhang, Q, “State of the Art and Current Trends on Layered Inorganic-Polymer Nanocomposite Coatings for Anticorrosion and Multi-Functional Applications.” Prog. Org. Coat., 163 106684. https://doi.org/10.1016/j.porgcoat.2021.106684 (2022)
Paredes, JI, Villar-Rodil, S, Martínez-Alonso, A, Tascón, JMD, “Graphene Oxide Dispersions in Organic Solvents.” Langmuir, 24 (19) 10560–10564. https://doi.org/10.1021/la801744a (2008)
Mkhoyan, KA, et al. “Atomic and Electronic Structure of Graphene-Oxide.” Nano Lett., 9 (3) 1058–1063. https://doi.org/10.1021/nl8034256 (2009)
Allahbakhsh, A, Sharif, F, Mazinani, S, “The Influence of Oxygen-Containing Functional Groups on the Surface Behavior and Roughness Characteristics of Graphene Oxide.” Nano, 8 (4) 1–8. https://doi.org/10.1142/S1793292013500458 (2013)
Inagaki, M, Kang, F, “Graphene Derivatives: Graphane, Fluorographene, Graphene Oxide, Graphyne and Graphdiyne.” J. Mater. Chem. A, 2 13193–13206. https://doi.org/10.1039/c4ta01183j (2014)
Sengupta, I, Chakraborty, S, Talukdar, M, Pal, SK, Chakraborty, S, “Thermal Reduction of Graphene Oxide: How Temperature Influences Purity.” J. Mater. Res., 33 (23) 4113–4122. https://doi.org/10.1557/jmr.2018.338 (2018)
Chung, C, Kim, Y, Shin, D, Ryoo, S, Hong, BHEE, Min, D, “Graphene Oxide.” (2012)
Cao, Y, et al. “Combination of TNF-α and Graphene Oxide-Loaded BEZ235 to Enhance Apoptosis of PIK3CA Mutant Colorectal Cancer Cells.” J. Mater. Chem. B, 1 (41) 5602–5610. https://doi.org/10.1039/c3tb20764a (2013)
Yoo, BM, Shin, HJ, Yoon, HW, Park, HB, “Graphene and Graphene Oxide and Their Uses in Barrier Polymers.” J. Appl. Polym. Sci., 131 (1) 1–23. https://doi.org/10.1002/app.39628 (2014)
Brodie, BC, “On the Atomic Weight of Graphite.” Philos. Trans. R. Soc. B Biol. Sci., 303 1–62. https://doi.org/10.1098/rstb.1983.0080 (1983)
Terrones, M, et al. “Interphases in Graphene Polymer-Based Nanocomposites: Achievements and Challenges.” Adv. Mater., 23 (44) 5302–5310. https://doi.org/10.1002/adma.201102036 (2011)
Poh, HL, Šaněk, F, Ambrosi, A, Zhao, G, Sofer, Z, Pumera, M, “Graphenes Prepared by Staudenmaier, Hofmann and Hummers Methods with Consequent Thermal Exfoliation Exhibit Very Different Electrochemical Properties.” Nanoscale, 4 (11) 3515–3522. https://doi.org/10.1039/c2nr30490b (2012)
Kovtyukhova, NI, Ollivier, PJ, Martin, BR, Mallouk, TE, Buzaneva, EV, Gorchinskiy, AD, “Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations.” Chem. Mater., 11 (3) 771–778. https://doi.org/10.1021/cm981085u (1999)
Moon, IK, Lee, J, Ruoff, RS, Lee, H, “Reduced Graphene Oxide by Chemical Graphitization.” Nat. Commun., 1 (6) 1–6. https://doi.org/10.1038/ncomms1067 (2010)
Pei, S, Cheng, HM, “The Reduction of Graphene Oxide.” Carbon N. Y., 50 (9) 3210–3228. https://doi.org/10.1016/j.carbon.2011.11.010 (2012)
Chen, S, et al. “Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy.” ACS Nano, 5 (2) 1321–1327. https://doi.org/10.1021/nn103028d (2011)
Prasai, D, Tuberquia, JC, Harl, RR, Jennings, GK, Bolotin, KI, “Graphene: Corrosion-Inhibiting Coating.” ACS Nano, 6 (2) 1102–1108. https://doi.org/10.1021/nn203507y (2012)
Böhm, S, “Graphene Against Corrosion.” Nat. Nanotechnol., 9 (10) 741–742. https://doi.org/10.1038/nnano.2014.220 (2014)
Raman, RKS, Tiwari, A, “Graphene: The Thinnest Known Coating for Corrosion Protection.” JOM, 66 (4) 637–642. https://doi.org/10.1007/s11837-014-0921-3 (2014)
Ye, X, Lin, Z, Zhang, H, Zhu, H, Liu, Z, Zhong, M, “Protecting Carbon Steel from Corrosion by Laser In Situ Grown Graphene Films.” Carbon N. Y., 94 326–334. https://doi.org/10.1016/j.carbon.2015.06.080 (2015)
Wang, B, Cunning, BV, Park, SY, Huang, M, Kim, JY, Ruoff, RS, “Graphene Coatings as Barrier Layers to Prevent the Water-Induced Corrosion of Silicate Glass.” ACS Nano, 10 (11) 9794–9800. https://doi.org/10.1021/acsnano.6b04363 (2016)
Zhu, M, et al. “Low-Temperature in Situ Growth of Graphene on Metallic Substrates and Its Application in Anticorrosion.” ACS Appl. Mater. Interfaces, 8 (1) 502–510. https://doi.org/10.1021/acsami.5b09453 (2016)
Huang, WH, Lin, CH, Lin, BS, Sun, CL, “Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties.” Materials (Basel), https://doi.org/10.3390/ma11101989 (2018)
Schriver, M, Regan, W, Gannett, WJ, Zaniewski, AM, Crommie, MF, Zettl, A, “Graphene as a Long-Term Metal Oxidation Barrier: Worse than Nothing.” ACS Nano, 7 (7) 5763–5768. https://doi.org/10.1021/nn4014356 (2013)
Zhou, F, Li, Z, Shenoy, GJ, Li, L, Liu, H, “Enhanced Room-Temperature Corrosion of Copper in the Presence of Graphene.” ACS Nano, 7 (8) 6939–6947. https://doi.org/10.1021/nn402150t (2013)
Wlasny, I, et al. “Role of Graphene Defects in Corrosion of Graphene-Coated Cu(111) Surface.” Appl. Phys. Lett., https://doi.org/10.1063/1.4795861 (2013)
Dong, Y, Liu, Q, Zhou, Q, “Corrosion Behavior of Cu During Graphene Growth by CVD.” Corros. Sci., 89 214–219. https://doi.org/10.1016/j.corsci.2014.08.026 (2014)
Stoot, AC, Camilli, L, Spiegelhauer, SA, Yu, F, Bøggild, P, “Multilayer Graphene for Long-Term Corrosion Protection of Stainless Steel Bipolar Plates for Polymer Electrolyte Membrane Fuel Cell.” J. Power Sources, 293 846–851. https://doi.org/10.1016/j.jpowsour.2015.06.009 (2015)
Ren, YJ, Anisur, MR, Qiu, W, He, JJ, Al-Saadi, S, Raman, RKS, “Degradation of Graphene Coated Copper in Simulated Proton Exchange Membrane Fuel Cell Environment: Electrochemical Impedance Spectroscopy Study.” J. Power Sources, 362 366–372. https://doi.org/10.1016/j.jpowsour.2017.07.041 (2017)
Ren, S, Cui, M, Li, W, Pu, J, Xue, Q, Wang, L, “N-doping of Graphene: Toward Long-term Corrosion Protection of Cu.” J. Mater. Chem. A, 6 24136–24148. https://doi.org/10.1039/C8TA05421E (2018)
H. Uǧuz et al., “ce pte d M us pt.” J. Phys. Energy, 2 (1) 0–31 (2020)
Chang, CH, et al. “Novel Anticorrosion Coatings Prepared from Polyaniline/Graphene Composites.” Carbon N. Y., 50 (14) 5044–5051. https://doi.org/10.1016/j.carbon.2012.06.043 (2012)
Li, Y, et al. “Self-aligned Graphene as Anticorrosive Barrier in Waterborne Polyurethane Composite Coatings.” J. Mater. Chem. A, 2 (34) 14139–14145. https://doi.org/10.1039/c4ta02262a (2014)
Röding, M, Gaska, K, Kádár, R, Lorén, N, “Computational Screening of Diffusive Transport in Nanoplatelet-Filled Composites: Use of Graphene to Enhance Polymer Barrier Properties.” ACS Appl. Nano Mater., 1 (1) 160–167. https://doi.org/10.1021/acsanm.7b00067 (2018)
Wei, J, Vo, T, Inam, F, “Epoxy/Graphene Nanocomposites - Processing and Properties: A Review.” RSC Adv., 5 (90) 73510–73524. https://doi.org/10.1039/c5ra13897c (2015)
Cui, Y, Kundalwal, SI, Kumar, S, “Gas Barrier Performance of Graphene/Polymer Nanocomposites.” Carbon N. Y., 98 313–333. https://doi.org/10.1016/j.carbon.2015.11.018 (2016)
Kim, H, Miura, Y, MacOsko, CW, “Graphene/Polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity.” Chem. Mater., 22 (11) 3441–3450. https://doi.org/10.1021/cm100477v (2010)
Putz, KW, Compton, OC, Palmeri, MJ, Nguyen, SBT, Brinson, LC, “High-nanofiller-Content Graphene Oxide-Polymer Nanocomposites via Vacuum-Assisted Self-Assembly.” Adv. Funct. Mater., 20 (19) 3322–3329. https://doi.org/10.1002/adfm.201000723 (2010)
Bortz, DR, Heras, EG, Martin-Gullon, I, “Impressive Fatigue Life and Fracture Toughness Improvements in Graphene Oxide/Epoxy Composites.” Macromolecules, 45 (1) 238–245 (2012)
Layek, RK, Das, AK, Park, MU, Kim, NH, Lee, JH, “Layer-Structured Graphene Oxide/Polyvinyl Alcohol Nanocomposites: Dramatic Enhancement of Hydrogen Gas Barrier Properties.” J. Mater. Chem. A, 2 (31) 12158–12161. https://doi.org/10.1039/c4ta02346c (2014)
Paul, DR, Robeson, LM, “Polymer Nanotechnology: Nanocomposites.” Polymer (Guildf), 49 (15) 3187–3204. https://doi.org/10.1016/j.polymer.2008.04.017 (2008)
Kim, H, Abdala, AA, MacOsko, CW, “Graphene/polymer Nanocomposites.” Macromolecules, 43 (16) 6515–6530. https://doi.org/10.1021/ma100572e (2010)
Potts, JR, Dreyer, DR, Bielawski, CW, Ruoff, RS, “Graphene-Based Polymer Nanocomposites.” Polymer (Guildf), 52 (1) 5–25. https://doi.org/10.1016/j.polymer.2010.11.042 (2011)
Kuilla, T, Bhadra, S, Yao, D, Kim, NH, Bose, S, Lee, JH, “Recent Advances in Graphene Based Polymer Composites.” Prog. Polym. Sci., 35 (11) 1350–1375. https://doi.org/10.1016/j.progpolymsci.2010.07.005 (2010)
Yousefi, N, et al. “Simultaneous In situ Reduction, Self-alignment and Covalent Bonding in Graphene Oxide/Epoxy Composites.” Carbon N. Y., 59 406–417. https://doi.org/10.1016/j.carbon.2013.03.034 (2013)
Ni, Y, et al. “Superior Mechanical Properties of Epoxy Composites Reinforced by 3D Interconnected Graphene Skeleton.” ACS Appl. Mater. Interfaces, 7 (21) 11583–11591. https://doi.org/10.1021/acsami.5b02552 (2015)
Cheng, J, Chen, S, Zhang, F, Shen, B, Lu, X, Pan, J, “Corrosion- and Wear-Resistant Composite Film of Graphene and Mussel Adhesive Proteins on Carbon Steel.” Corros. Sci., 164 108351. https://doi.org/10.1016/j.corsci.2019.108351 (2020)
Liu, S, Gu, L, Zhao, H, Chen, J, Yu, H, “Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings.” J. Mater. Sci. Technol., 32 (5) 425–431. https://doi.org/10.1016/j.jmst.2015.12.017 (2016)
Chen, C, et al. “Achieving High Performance Corrosion and Wear Resistant Epoxy Coatings via Incorporation of Noncovalent Functionalized Graphene.” Carbon N. Y., 114 356–366. https://doi.org/10.1016/j.carbon.2016.12.044 (2017)
Cui, M, Ren, S, Pu, J, Wang, Y, Zhao, H, Wang, L, “Poly(o-phenylenediamine) Modified Graphene Toward the Reinforcement in Corrosion Protection of Epoxy Coatings.” Corros. Sci., 159 108131. https://doi.org/10.1016/j.corsci.2019.108131 (2019)
Qiu, S, Li, W, Zheng, W, Zhao, H, Wang, L, “Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% nacl Solution.” ACS Appl. Mater. Interfaces, 9 (39) 34294–34304. https://doi.org/10.1021/acsami.7b08325 (2017)
Ye, Y, et al. “Superior Corrosion Resistance and Self-Healable Epoxy Coating Pigmented with Silanzied Trianiline-Intercalated Graphene.” Carbon N. Y., 142 164–176. https://doi.org/10.1016/j.carbon.2018.10.050 (2019)
Cui, Y, Kundalwal, SI, Kumar, S, “Gas Barrier Performance of Graphene/Polymer Nanocomposites.” Carbon N. Y., 98 313–333. https://doi.org/10.1016/j.carbon.2015.11.018 (2016)
Jiao, W, et al. “Improving the Gas Barrier Properties of Fe3O4/Graphite Nanoplatelet Reinforced Nanocomposites by a Low Magnetic Field Induced Alignment.” Compos. Sci. Technol., 99 124–130. https://doi.org/10.1016/j.compscitech.2014.05.022 (2014)
Li, X, Bandyopadhyay, P, Guo, M, Kim, NH, Lee, JH, “Enhanced Gas Barrier and Anticorrosion Performance of Boric Acid Induced Cross-linked Poly(vinyl alcohol-co-ethylene)/Graphene Oxide Film.” Carbon N. Y., 133 150–161. https://doi.org/10.1016/j.carbon.2018.03.036 (2018)
Tamilarasan, TR, Sanjith, U, Shankar, MS, Rajagopal, G, “Effect of Reduced Graphene Oxide (rGO) on Corrosion and Erosion-Corrosion Behaviour of Electroless Ni-P Coatings.” Wear, 390 385–391. https://doi.org/10.1016/j.wear.2017.09.004 (2017)
Rana, ARK, Islam, MA, Farhat, Z, “Effect of Graphene Nanoplatelets (GNPs) Addition on Erosion-Corrosion Resistance of Electroless Ni–P Coatings.” J. Bio-Tribo-Corros., https://doi.org/10.1007/s40735-019-0304-y (2020)
Sun, W, et al. “Inhibiting the Corrosion-Promotion Activity of Graphene.” Chem. Mater., 27 (7) 2367–2373. https://doi.org/10.1021/cm5043099 (2015)
Ramezanzadeh, B, Moghadam, MHM, Shohani, N, Mahdavian, M, “Effects of Highly Crystalline and Conductive Polyaniline/Graphene Oxide Composites on the Corrosion Protection Performance of a Zinc-Rich Epoxy Coating.” Chem. Eng. J., 320 363–375. https://doi.org/10.1016/j.cej.2017.03.061 (2017)
Ding, R, Wang, X, Jiang, J, Gui, T, Li, W, “Study on Evolution of Coating State and Role of Graphene in Graphene-Modified Low-Zinc Waterborne Epoxy Anticorrosion Coating by Electrochemical Impedance Spectroscopy.” J. Mater. Eng. Perform., 26 (7) 3319–3335. https://doi.org/10.1007/s11665-017-2790-8 (2017)
Ding, R, Zheng, Y, Yu, H, Li, W, Wang, X, Gui, T, “Study of Water Permeation Dynamics and Anti-Corrosion Mechanism of Graphene/Zinc Coatings.” J. Alloys Compd., 748 481–495. https://doi.org/10.1016/j.jallcom.2018.03.160 (2018)
Ding, R, et al. “The Diffusion-Dynamical and Electrochemical Effect Mechanism of Oriented Magnetic Graphene on Zinc-Rich Coatings and the Electrodynamics and Quantum Mechanics Mechanism of Electron Conduction in Graphene Zinc-Rich Coatings.” J. Alloys Compd., 784 756–768. https://doi.org/10.1016/j.jallcom.2019.01.070 (2019)
Motamedi, M, Ramezanzadeh, M, Ramezanzadeh, B, Saadatmandi, S, “Enhancement of the Active/Passive Anti-Corrosion Properties of Epoxy Coating via Inclusion of Histamine/Zinc Modified/Reduced Graphene Oxide Nanosheets.” Appl. Surf. Sci., 488 77–91. https://doi.org/10.1016/j.apsusc.2019.05.180 (2019)
Taheri, NN, Ramezanzadeh, B, Mahdavian, M, “Application of Layer-by-layer Assembled Graphene Oxide Nanosheets/Polyaniline/Zinc Cations for Construction of an Effective Epoxy Coating Anti-Corrosion System.” J. Alloys Compd., 800 532–549. https://doi.org/10.1016/j.jallcom.2019.06.103 (2019)
Zhu, Q, et al. “Synergistic Effect of Polypyrrole Functionalized Graphene Oxide and Zinc Phosphate for Enhanced Anticorrosion Performance of Epoxy Coatings.” Compos. Part A Appl. Sci. Manuf., 130 2020. https://doi.org/10.1016/j.compositesa.2019.105752 (2019)
Ramezanzadeh, M, Bahlakeh, G, Ramezanzadeh, B, “Green Synthesis of Reduced Graphene Oxide Nanosheets Decorated with Zinc-Centered Metal-Organic Film for Epoxy-Ester Composite Coating Reinforcement: DFT-D Modeling and Experimental Explorations.” J. Taiwan Inst. Chem. Eng., 114 311–330. https://doi.org/10.1016/j.jtice.2020.09.003 (2020)
Bai, W, Ma, Y, Meng, M, Li, Y, “The Influence of Graphene on the Cathodic Protection Performance of Zinc-Rich Epoxy Coatings.” Prog. Org. Coat., 161 106. https://doi.org/10.1016/j.porgcoat.2021.106456 (2021)
Yu, YH, Lin, YY, Lin, CH, Chan, CC, Huang, YC, “High-performance Polystyrene/Graphene-Based Nanocomposites with Excellent Anti-corrosion Properties.” Polym. Chem., 5 (2) 535 (2014)
Fayyad, EM, Sadasivuni, KK, Ponnamma, D, Al-Maadeed, MAA, “Oleic Acid-grafted Chitosan/Graphene Oxide Composite Coating for Corrosion Protection of Carbon Steel.” Carbohydr. Polym., 151 871–878. https://doi.org/10.1016/j.carbpol.2016.06.001 (2016)
Sheng, X, Cai, W, Zhong, L, Xie, D, Zhang, X, “Synthesis of Functionalized Graphene/Polyaniline Nanocomposites with Effective Synergistic Reinforcement on Anticorrosion.” Ind. Eng. Chem. Res., 55 (31) 8576–8585. https://doi.org/10.1021/acs.iecr.6b01975 (2016)
Li, J, Cui, J, Yang, J, Li, Y, Qiu, H, Yang, J, “Reinforcement of Graphene and Its Derivatives on the Anticorrosive Properties of Waterborne Polyurethane Coatings.” Compos. Sci. Technol., 129 30–37. https://doi.org/10.1016/j.compscitech.2016.04.017 (2016)
Qi, K, Sun, Y, Duan, H, Guo, X, “A Corrosion-Protective Coating Based on a Solution-Processable Polymer-Grafted Graphene Oxide Nanocomposite.” Corros. Sci., 98 500–506. https://doi.org/10.1016/j.corsci.2015.05.056 (2015)
Yang, Z, Sun, W, Wang, L, Li, S, Zhu, T, Liu, G, “Liquid-Phase Exfoliated Fluorographene as a Two Dimensional Coating Filler for Enhanced Corrosion Protection Performance.” Corros. Sci., 103 312–318. https://doi.org/10.1016/j.corsci.2015.10.039 (2016)
Jafari, Y, Ghoreishi, SM, Shabani-Nooshabadi, M, “Electrochemical Deposition and Characterization of Polyaniline-Graphene Nanocomposite Films and Its Corrosion Protection Properties.” J. Polym. Res., https://doi.org/10.1007/s10965-016-0983-8 (2016)
Zhu, K, Li, X, Wang, H, Li, J, Fei, G, “Electrochemical and Anti-corrosion Behaviors of Water Dispersible Graphene/Acrylic Modified Alkyd Resin Latex Composites Coated Carbon Steel.” J. Appl. Polym. Sci., 134 (11) 1–12. https://doi.org/10.1002/app.44445 (2017)
Pourhashem, S, Vaezi, MR, Rashidi, A, “Investigating the Effect of SiO2-Graphene Oxide Hybrid as Inorganic Nanofiller on Corrosion Protection Properties of Epoxy Coatings.” Surf. Coat. Technol., 311 282–294. https://doi.org/10.1016/j.surfcoat.2017.01.013 (2017)
Zheng, H, Shao, Y, Wang, Y, Meng, G, Liu, B, “Reinforcing the Corrosion Protection Property of Epoxy Coating by Using Graphene Oxide–Poly(urea–formaldehyde) Composites.” Corros. Sci., 123 267–277. https://doi.org/10.1016/j.corsci.2017.04.019 (2017)
Hikku, GS, Jeyasubramanian, K, Venugopal, A, Ghosh, R, “Corrosion Resistance Behaviour of Graphene/Polyvinyl Alcohol Nanocomposite Coating for Aluminium-2219 Alloy.” J. Alloys Compd., 716 259–269. https://doi.org/10.1016/j.jallcom.2017.04.324 (2017)
Hayatdavoudi, H, Rahsepar, M, “A Mechanistic Study of the Enhanced Cathodic Protection Performance of Graphene-Reinforced Zinc Rich Nanocomposite Coating for Corrosion Protection of Carbon Steel Substrate.” J. Alloys Compd., 727 1148–1156. https://doi.org/10.1016/j.jallcom.2017.08.250 (2017)
Pourhashem, S, Vaezi, MR, Rashidi, A, Bagherzadeh, MR, “Exploring Corrosion Protection Properties of Solvent Based Epoxy-Graphene Oxide Nanocomposite Coatings on Mild Steel.” Corros. Sci., 115 78–92. https://doi.org/10.1016/j.corsci.2016.11.008 (2017)
Harfouche, N, Gospodinova, N, Nessark, B, Perrin, FX, “Electrodeposition of Composite Films of Reduced Graphene Oxide/Polyaniline in Neutral Aqueous Solution on Inert and Oxidizable Metal.” J. Electroanal. Chem., 786 135–144. https://doi.org/10.1016/j.jelechem.2017.01.030 (2017)
Tong, Y, Bohm, S, Song, M, “The Capability of Graphene on Improving the Electrical Conductivity and Anti-Corrosion Properties of Polyurethane Coatings.” Appl. Surf. Sci., 424 72–81. https://doi.org/10.1016/j.apsusc.2017.02.081 (2017)
Nayak, SR, Mohana, KNS, “Corrosion Protection Performance of Functionalized Graphene Oxide Nanocomposite Coating on Mild Steel.” Surf. Interfaces, 11 63–73. https://doi.org/10.1016/j.surfin.2018.03.002 (2018)
Kim, H, Lee, H, Lim, HR, Cho, HB, Choa, YH, “Electrically Conductive and Anti-Corrosive Coating on Copper Foil Assisted By Polymer-Nanocomposites Embedded with Graphene.” Appl. Surf. Sci., 476 123–127. https://doi.org/10.1016/j.apsusc.2019.01.066 (2019)
Ziat, Y, Hammi, M, Zarhri, Z, Laghlimi, C, “Epoxy Coating Modified with Graphene: A Promising Composite Against Corrosion Behavior of Copper Surface in Marine Media.” J. Alloys Compd., 820 153380. https://doi.org/10.1016/j.jallcom.2019.153380 (2020)
Li, H, et al. “The Synergistic Effect of Polyvinyl Alcohol and Graphene Oxide on the Corrosion Resistance of Waterborne Coatings.” Prog. Org. Coat., 160 106526. https://doi.org/10.1016/j.porgcoat.2021.106526 (2021)
Liu, S, et al. “A Facile Approach to Fabricating Graphene/Waterborne Epoxy Coatings with Dual Functionalities of Barrier and Corrosion Inhibitor.” J. Mater. Sci. Technol., 112 263–276. https://doi.org/10.1016/j.jmst.2021.07.061 (2021)
Araujo, AF, Ferreira, MVF, Felisberto, MDV, Sicupira, DC, Santos, A, “Progress in Organic Coatings Corrosion Resistance of a Superelastic NiTi Alloy Coated with Graphene–Based Coatings.” Prog. Org. Coat., 165 106727. https://doi.org/10.1016/j.porgcoat.2022.106727 (2022)
Liu, S, et al. “A Facile Approach to Fabricating Graphene/Waterborne Epoxy Coatings with Dual Functionalities of Barrier and Corrosion Inhibitor.” J. Mater. Sci. Technol., 112 263–276. https://doi.org/10.1016/j.jmst.2021.07.061 (2022)
Zhao, Z, Zhou, M, Zhao, W, Hu, J, Fu, H, “Anti-corrosion Epoxy / Modified Graphene Oxide / Glass Fiber Composite Coating with Dual Physical Barrier Network.” Prog. Org. Coat., 167 1068. https://doi.org/10.1016/j.porgcoat.2022.106823 (2022)
Liu, Z, et al. “Integrated Dual-Functional ORMOSIL Coatings with AgNPs@rGO Nanocomposite for Corrosion Resistance and Antifouling Applications.” ACS Sustain. Chem. Eng., 8 (17) 6786–6797. https://doi.org/10.1021/acssuschemeng.0c01294 (2020)
Jin, H, Tian, L, Bing, W, Zhao, J, Ren, L, “Toward the Application of Graphene for Combating Marine Biofouling.” Adv. Sustain. Syst., 5 (1) 1–16. https://doi.org/10.1002/adsu.202000076 (2021)
Jena, G, Sofia, S, Anandkumar, B, Vanithakumari, SC, George, RP, Philip, J, “Graphene Oxide/Polyvinylpyrrolidone Composite Coating on 316L SS with Superior Antibacterial and Anti-Biofouling Properties.” Prog. Org. Coat., 158 106356. https://doi.org/10.1016/j.porgcoat.2021.106356 (2021)
Selim, MS, Fatthallah, NA, Higazy, SA, Hao, Z, Mo, PJ, “A Comparative Study Between Two Novel Silicone/Graphene-Based Nanostructured Surfaces for Maritime Antifouling.” J. Colloid Interface Sci., 606 367–383. https://doi.org/10.1016/j.jcis.2021.08.026 (2022)
Selim, MS, Azzam, AM, Higazy, SA, El-safty, SA, Shenashen, MA, “Progress in Organic Coatings Novel Graphene-Based Ternary Nanocomposite Coatings as Ecofriendly Antifouling Brush Surfaces.” Prog. Org. Coat., 167 1068. https://doi.org/10.1016/j.porgcoat.2022.106803 (2022)
Tseng, IH, Liao, YF, Chiang, JC, Tsai, MH, “Transparent Polyimide/Graphene Oxide Nanocomposite with Improved Moisture Barrier Property.” Mater. Chem. Phys., 136 (1) 247–253. https://doi.org/10.1016/j.matchemphys.2012.06.061 (2012)
Tissera, ND, Wijesena, RN, Perera, JR, De Silva, KMN, Amaratunge, GAJ, “Hydrophobic Cotton Textile Surfaces Using an Amphiphilic Graphene Oxide (GO) Coating.” Appl. Surf. Sci., 324 455–463. https://doi.org/10.1016/j.apsusc.2014.10.148 (2015)
Shokrieh, MM, Hosseinkhani, MR, Naimi-Jamal, MR, Tourani, H, “Nanoindentation and Nanoscratch Investigations on Graphene-Based Nanocomposites.” Polym. Test., 32 (1) 45–51. https://doi.org/10.1016/j.polymertesting.2012.09.001 (2013)
Sharmila, TKB, Antony, JV, Jayakrishnan, MP, Beegum, PMS, Thachil, ET, “Mechanical, Thermal and Dielectric Properties of Hybrid Composites of Epoxy and Reduced Graphene Oxide/Iron Oxide.” Mater. Des., 90 66–75. https://doi.org/10.1016/j.matdes.2015.10.055 (2016)
Salom, C, Prolongo, MG, Toribio, A, Martínez-Martínez, AJ, de Cárcer, IA, Prolongo, SG, “Mechanical Properties and Adhesive Behavior of Epoxy-Graphene Nanocomposites.” Int. J. Adhes. Adhes., 84 119–125. https://doi.org/10.1016/j.ijadhadh.2017.12.004 (2018)
Haghdadeh, P, Ghaffari, M, Ramezanzadeh, B, Bahlakeh, G, Saeb, MR, “The Role of Functionalized Graphene Oxide on the Mechanical and Anti-Corrosion Properties of Polyurethane Coating.” J. Taiwan Inst. Chem. Eng., 86 199–212. https://doi.org/10.1016/j.jtice.2018.02.009 (2018)
Chakraborty, G, Valapa, RB, Pugazhenthi, G, Katiyar, V, “Investigating the Properties of Poly (lactic acid)/exfoliated Graphene Based Nanocomposites Fabricated by Versatile Coating Approach.” Int. J. Biol. Macromol., 113 (2017) 1080–1091. https://doi.org/10.1016/j.ijbiomac.2018.03.037 (2018)
Hasani, M, Mahdavian, M, Yari, H, Ramezanzadeh, B, “Versatile Protection of Exterior Coatings by the Aid of Graphene Oxide Nano-Sheets; Comparison with Conventional UV Absorbers.” Prog. Org. Coat., 116 90–101. https://doi.org/10.1016/j.porgcoat.2017.11.020 (2018)
Lu, T, Solis-Ramos, E, Yi, Y, Kumosa, M, “UV Degradation Model for Polymers and Polymer Matrix Composites.” Polym. Degrad. Stab., 154 203–210. https://doi.org/10.1016/j.polymdegradstab.2018.06.004 (2018)
Dong, B, Yuan, Y, Luo, J, Dong, L, Liu, R, Liu, X, “Acryloyl-Group Functionalized Graphene for Enhancing Thermal and Mechanical Properties of Acrylated Epoxidized Soybean Oil UV-Curable Based Coatings.” Prog. Org. Coat., 118 57–65. https://doi.org/10.1016/j.porgcoat.2018.01.020 (2018)
Othman, NH, Ismail, MC, Mustapha, M, Sallih, N, Kee, KE, Jaal, RA, “Graphene-Based Polymer Nanocomposites as Barrier Coatings for Corrosion Protection.” Prog. Org. Coat., 135 82–99. https://doi.org/10.1016/j.porgcoat.2019.05.030 (2019)
Acknowledgments
The authors are highly thankful to the Department of Mechanical Engineering, Dr. BR Ambedkar National Institute of Technology Jalandhar for providing the facilities for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons. The data will be made available on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Sharma, S. Graphene-based polymer coatings for preventing marine corrosion: a review. J Coat Technol Res 20, 413–432 (2023). https://doi.org/10.1007/s11998-022-00730-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00730-x