Abstract
There is an increase in public awareness of functional textiles, which strive to produce innovative fabrics for specific applications. In this context, acrylic polymer, a significant synthetic fabric used in the textile industry, was selected to provide it with multifunctional characteristics. For this purpose, the acrylic fabric was functionalized with amidoxime groups to serve as ligands for immobilizing silver ions so that it can be further in situ reduced to AgNPs. Polydopamine (PDA) has the advantage of being an ion binding and reducing agent by virtue of its phenolic hydroxyl groups capable of reducing silver ions to AgNPs, and it was exploited in this study for imparting the acrylic fabric with antimicrobial and antioxidant properties. Additionally, glucose as a reducing agent was also used to form AgNPs-loaded amidoximated acrylic fabric. The pristine, amidoximated, PDA-coated AgNPs-loaded amidoximated, and AgNPs-loaded amidoximated acrylic fabrics have been characterized using ATR-FTIR, SEM, EDX, and tensile strength. All fabric samples were assessed as antioxidants using DPPH and FRAP assays. Antimicrobial properties against Pseudomonas Aeruginosa, Staphylococcus aureus, Escherichia coli and Candida albicans were also evaluated using broth assay. An almost complete killing (99.99%) of the pathogenic Gram-negative Escherichia coli was achieved using PDA-coated AgNPs-loaded amidoximated acrylic fabric. The overall results indicated that the composite nanocoating using PDA-AgNPs showed good tensile strength and excellent antioxidant and antimicrobial properties to suggest the viability of PDA-AgNPs coating of fabrics for possible application as biomedical textiles.
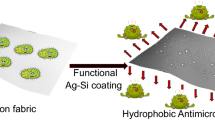
Graphical abstract








Similar content being viewed by others
References
Mishra, A, Sharma, S, Gupta, B, “Studies on the Amidoximation of Polyacrylonitrile Films: Influence of Synthesis Conditions.” Journal of Applied Polymer Science, 121 (5) 2705–2709 (2011)
Jamil, SNAM, Daik, R, Ahmad, I, “Redox Copolymerization of Acrylonitrile with Fumaronitrile as a Precursor for Carbon Fibre.” Journal of Polymer Research, 14 (5) 379–385 (2007)
Mohamed, SA, Al-Ghamdi, SS, El-Shishtawy, RM, “Immobilization of Horseradish Peroxidase on Amidoximated Acrylic Polymer Activated by Cyanuric Chloride.” International Journal of Biological Macromolecules, 91 663–670 (2016)
Almulaiky, YQ, Aqlan, FM, Aldhahri, M, Baeshen, M, Khan, TJ, Khan, KA, Alayafi, AA, “α-Amylase Immobilization on Amidoximated Acrylic Microfibres Activated by Cyanuric Chloride.” Royal Society Open Science, 5 (11) 172164 (2018)
Kiani, GR, Sheikhloie, H, Arsalani, N, “Heavy Metal Ion Removal from Aqueous Solutions by Functionalized Polyacrylonitrile.” Desalination, 269 (1–3) 266–270 (2011)
Liu, X, Chen, H, Wang, C, Qu, R, Ji, C, Sun, C, Zhang, Y, “Synthesis of Porous Acrylonitrile/Methyl Acrylate Copolymer Beads by Suspended Emulsion Polymerization and Their Adsorption Properties After Amidoximation.” Journal of Hazardous Materials, 175 (1–3) 1014–1021 (2010)
Choi, SH, Nho, YC, “Adsorption of UO2+2 by Polyethylene Adsorbents with Amidoxime, Carboxyl, and Amidoxime/Carboxyl Group.” Radiation Physics and Chemistry, 57 (2) 187–193 (2000)
Shaaban, AF, Fadel, DA, Mahmoud, AA, Elkomy, MA, Elbahy, SM, “Synthesis of a New Chelating Resin Bearing Amidoxime Group for Adsorption of Cu(II), Ni(II) and Pb(II) by Batch and Fixed-Bed Column Methods.” Journal of Environmental Chemical Engineering, 2 (1) 632–641 (2014)
Ge, F, Li, MM, Ye, H, Zhao, BX, “Effective Removal of Heavy Metal Ions Cd2+, Zn2+, Pb2+, Cu2+ from Aqueous Solution by Polymer-Modified Magnetic Nanoparticles.” Journal of Hazardous Materials, 211 366–372 (2012)
Mou, NI, Tabib-Azar, M, “Photoreduction of Ag+ in Ag/Ag2S/Au Memristor.” Applied Surface Science, 340 138–142 (2015)
Rubina, MS, Kamitov, EE, Zubavichus, YaV, Peters, GS, Naumkin, AV, Suzer, S, Vasil’kova, AYu, “Collagen-Chitosan Scaffold Modified with Au and Ag Nanoparticles: Synthesis and Structure.” Applied Surface Science, 366 365–371 (2016)
Hu, L, Kang, Z, “Enhanced Flexible Polypropylene Fabric with Silver/Magnetic Carbon Nanotubes Coatings for Electromagnetic Interference Shielding.” Applied Surface Science, 568 150845 (2021)
Karki, HP, Ojha, DP, Joshi, MK, Kim, HJ, “Effective Reduction of p-Nitrophenol by Silver Nanoparticle Loaded on Magnetic Fe3O4/ATO Nano-Composite.” Applied Surface Science, 435 599–608 (2018)
Hu, W, Chen, S, Li, X, Shi, S, Shen, W, Zhang, X, Wang, H, “In Situ Synthesis of Silver Chloride Nanoparticles into Bacterial Cellulose Membranes.” Materials Science and Engineering: C, 29 (4) 1216–1219 (2009)
Ma, Y, Zhou, T, Zhao, C, “Preparation of Chitosan–Nylon-6 Blended Membranes Containing Silver Ions as Antibacterial Materials.” Carbohydrate Research, 343 (2) 230–237 (2008)
Salam, MA, Obaid, AY, El-Shishtawy, RM, Mohamed, SA, “Synthesis of Nanocomposites of Polypyrrole/Carbon Nanotubes/Silver Nano Particles and Their Application in Water Disinfection.” RSC Advances, 7 (27) 16878–16884 (2017)
Chen, CY, Chiang, CL, “Preparation of Cotton Fibers with Antibacterial Silver Nanoparticles.” Materials Letters, 62 (21–22) 3607–3609 (2008)
Perelshtein, I, Applerot, G, Perkas, N, Wehrschuetz-Sigl, E, Hasmann, A, Gübitz, G, Gedanken, A, “CuO–Cotton Nanocomposite: Formation, Morphology, and Antibacterial Activity.” Surface and Coatings Technology, 204 (1–2) 54–57 (2009)
El-Shishtawy, RM, Asiri, AM, Abdelwahed, NAM, Al-Otaibi, MM, “In Situ Production of Silver Nanoparticle on Cotton Fabric and Its Antimicrobial evaluation.” Cellulose, 18 (1) 75–82 (2011)
Ahmed, NSE, “Color Data and Antibacterial Properties of Smart Ag/Polypyrrole-Nanocoated Cotton Fabric.” Egyptian Journal of Chemistry, 63 (9) 3547–3555 (2020)
Shabbir, M, Mohammad, F, “Multifunctional AgNPs@Wool: Colored, UV-Protective and Antioxidant Functional Textiles.” Applied Nanoscience, 8 545–555 (2018)
Guo, S, Yu, B, Ahmed, A, Cong, H, Shen, Y, “Synthesis of Polyacrylonitrile/Polytetrahydropyrimidine (PAN/PTHP) Nanofibers with Enhanced Antibacterial and Anti-Viral Activities for Personal Protective Equipment.” Journal of Hazardous Materials, 424 127602 (2022)
Kim, YH, Lee, DK, Jo, BG, Jeong, JH, Kang, YS, “Synthesis of Oleate Capped Cu Nanoparticles by Thermal Decomposition.” Colloids and Surfaces A: Physicochemical and Engineering Aspects, 284 364–368 (2006)
Zhu, H, Zhang, C, Yin, Y, “Novel Synthesis of Copper Nanoparticles: Influence of the Synthesis Conditions on the Particle Size.” Nanotechnology, 16 (12) 3079 (2005)
Joshi, SS, Patil, SF, Iyer, V, Mahumuni, S, “Radiation Induced Synthesis and Characterization of Copper Nanoparticles.” Nanostructured Materials, 10 (7) 1135–1144 (1998)
Pileni, MP, Ninham, BW, Gulik-Krzywicki, T, Tanori, J, Lisiecki, I, Filankembo, A, “Direct Relationship Between Shape and Size of Template and Synthesis of Copper Metal Particles.” Advanced Materials, 11 (16) 1358–1362 (1999)
Husein, MM, Rodil, E, Vera, JH, “A Novel Method for the Preparation of Silver Chloride Nanoparticles Starting from Their Solid Powder Using Microemulsions.” Journal of Colloid and Interface Science, 288 (2) 457–467 (2005)
Lee, H, Dellatore, SM, Miller, WM, Messersmith, PB, “Mussel-Inspired Surface Chemistry for Multifunctional Coatings.” Science, 318 (5849) 426–430 (2007)
Waite, JH, Tanzer, ML, “Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline.” Science, 212 (4498) 1038–1040 (1981)
Waite, JH, Housley, TJ, Tanzer, ML, “Peptide Repeats in a Mussel Glue Protein: Theme and Variations.” Biochemistry, 24 (19) 5010–5014 (1985)
Foti, MC, Daquino, C, Geraci, C, “Electron-Transfer Reaction of Cinnamic Acids and their Methyl Esters with the DPPH•Radical in Alcoholic Solutions.” Journal of Organic Chemistry, 69 2309–2314 (2004)
Benzie, IF, Strain, JJ, “The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay.” Analytical Biochemistry, 239 (1) 70–76 (1996)
El-Shishtawy, RM, Ahmed, NSE, “Anionic Coloration of Acrylic Fibre. Part 1: Efficient Pretreatment and Dyeing with Acid Dyes.” Coloration Technology, 121 139–146 (2005)
Liu, M, Jiang, W, Chen, Q, Wang, S, Mao, Y, Gong, X, Leung, KC-F, Wang, H, Tian, J, Xuan, S, “A Facile One-Step Method to Synthesize SiO2@Polydopamine Core–Shell Nanospheres for Shear Thickening Fluid.” RSC Advances, 6 (35) 29279–29287 (2016)
Chen, L, Ma, J, Li, X, Zhang, J, Fang, J, Guan, Y, Xie, P, “Strong Enhancement on Fenton Oxidation by Addition of Hydroxylamine to Accelerate the Ferric and Ferrous Iron Cycles.” Environmental Science and Technology, 45 3925–3930 (2011)
Choukairi, Z, Hazzaz, T, José, MF, Fechtali, T, “The Cytotoxic Activity of Salvia officinalis L. and Rosmarinus officinalis L. Leaves Extracts on Human Glioblastoma Cell Line and Their Antioxidant Effect.” Journal of Complementary and Integrative Medicine, 17 (4) 20180189 (2020)
AL Zahrani, NA, El-Shishtawy, RM, Asiri, AM, “Recent Developments of Gallic Acid Derivatives and Their Hybrids in Medicinal Chemistry: A Review.” European Journal of Medicinal Chemistry, 204 112609 (2020)
Al Zahrani, NA, El-Shishtawy, RM, Elaasser, MM, Asiri, AM, “Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents.” Molecules, 25 (19) 4566 (2020)
Zhang, L, Luo, J, Menkhaus, TJ, Varadaraju, H, Sun, Y, Fong, H, “Antimicrobial Nano-Fibrous Membranes Developed from Electrospun Polyacrylonitrile Nanofibers.” Journal of Membrane Science, 369 499–505 (2011)
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: 19-SCI-1-02-0001.
Author information
Authors and Affiliations
Contributions
ESA: Project administration, Writing–review & editing. YQA: Synthesis, characterization, Writing–review & editing. SAA-H: Project administration, Writing–review & editing. RME-S: Conceptualization, Formal analysis, Writing the manuscript, Data curation and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allehyani, E.S., Almulaiky, Y.Q., Al-Harbi, S.A. et al. Polydopamine-AgNPs coated acrylic fabric for antimicrobial and antioxidant textiles. J Coat Technol Res 20, 1133–1143 (2023). https://doi.org/10.1007/s11998-022-00711-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00711-0