Abstract
Purpose of review
In this review, we review the current understanding of the pathogenesis and known associations in thyroid eye disease. We describe recent developments in treatment paradigms including recently approved agents, upcoming future therapies, and current technologies and strategies in approaching patients surgically.
Recent findings
The development of biologic agents, such as rituximab, tocilizumab, and most recently the human monoclonal antibody teprotumumab that binds to the IFG-1 receptor, has altered the management of patients in the active phase of thyroid eye disease. Randomized controlled trials have demonstrated teprotumumab infusion in the relatively early phase of orbital inflammation results in a durable reduction in proptosis and strabismus in the majority of patients. Regarding surgical management, many studies have described refining surgical techniques and approaches, with specific attention to customizing approaches based on pre-operative assessments and using computer-assisted technologies.
Summary
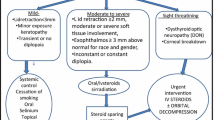
New insights in gene expression have improved our understanding of the etiology of thyroid eye disease and helped to define the role of lifestyle modifications. Steroids continue to have a role in temporizing severe orbitopathy while teprotumumab is useful in patients with moderate disease activity when utilized within 9 months of disease onset. Advances/progress in surgical management continue to increase effectiveness and safety through an ideal, predictable, consistent approach remains elusive.
Similar content being viewed by others
References
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38.
Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588.
Chin YH, Ng CH, Lee MH, Koh JWH, Kiew J, Yang SP, et al. Prevalence of thyroid eye disease in Graves’ disease: a meta-analysis and systematic review. Clin Endocrinol. 2020;93:363–74. https://doi.org/10.1111/cen.14296.
Nunery WR. Michael J. Hawes Lecture: Observations after forty years of managing thyroid eye disease – the importance of subtypes. Presented at: American Society of Ophthalmic Plastic and Reconstructive Surgery meeting; Oct. 10–11, 2019; San Francisco.
Choudhary MM, Zhang KR, Johnson S, Hwang CJ, Chon BH, Perry JD. Temporal fat pad volume in patients with thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;36:194–7.
Freitag SK, Tanking T. A nomenclature to describe the sequence of visual field defects in progressive thyroid eye disease-compressive optic neuropathy (an American Ophthalmological Society thesis). Am J Ophthalmol. 2020;213:293–305.
Tran AQ, Zhang-Nunes SX, Cahill K, et al. Thyroid eye disease with choroidal folds. Orbit. 2020:1–9.
Francis N, Francis T, Lazarus JH, Okosieme OE. Current controversies in the management of Graves’ hyperthyroidism. Expert Rev Endocrinol Metab. 2020;15:159–69.
Rosetti S, Tanda ML, Veronesi G, Masiello E, Premoli P, Gallo D, et al. Oral steroid prophylaxis for Graves’ orbitopathy after radioactive iodine treatment for Graves’ disease is not only effective, but also safe. J Endocrinol Investig. 2020;43:381–3.
Antonelli A, Ferrari SM, Ragusa F, Elia G, Paparo SR, Ruffilli I, et al. Graves’ disease: epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34:101387.
Wémeau J-L, Klein M, Sadoul J-L, Briet C, Vélayoudom-Céphise F-L. Graves’ disease: introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol (Paris). 2018;79:599–607.
Mishra S, Maurya VK, Kumar S, Kaur A, Saxena SK. Clinical management and therapeutic strategies for the thyroid-associated ophthalmopathy: current and future perspectives. Curr Eye Res. 2020:1–17.
Cawood TJ, Moriarty P, O’Farrelly C, O’Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92:59–64.
Vink JM, Jansen R, Brooks A, Willemsen G, van Grootheest G, de Geus E, et al. Differential gene expression patterns between smokers and non-smokers: cause or consequence? Addict Biol. 2017;22:550–60.
Hayashi M, Futawaka K, Matsushita M, Hatai M, Yoshikawa N, Nakamura K, et al. Cigarette smoke extract disrupts transcriptional activities mediated by thyroid hormones and its receptors. Biol Pharm Bull. 2018;41:383–93.
Chng CL, Lai OF, Chew CS-M, Peh YP, Fook-Chong SM-C, Seah LL, et al. Hypoxia increases adipogenesis and affects adipocytokine production in orbital fibroblasts-a possible explanation of the link between smoking and Graves’ ophthalmopathy. Int J Ophthalmol. 2014;7:403–7.
Planck T, Shahida B, Parikh H, Ström K, Åsman P, Brorson H, et al. Smoking induces overexpression of immediate early genes in active Graves’ ophthalmopathy. Thyroid. 2014;24:1524–32.
Kau H-C, Wu S-B, Tsai C-C, Liu CJ-L, Wei Y-H. Cigarette smoke extract-induced oxidative stress and fibrosis-related genes expression in orbital fibroblasts from patients with Graves’ ophthalmopathy. Oxidative Med Cell Longev. 2016;2016:4676289.
Hikage F, Atkins S, Kahana A, Smith TJ, Chun T-H. HIF2A-LOX pathway promotes fibrotic tissue remodeling in thyroid-associated orbitopathy. Endocrinology. 2019;160:20–35.
Sabini E, Mazzi B, Profilo MA, Mautone T, Casini G, Rocchi R, et al. High serum cholesterol is a novel risk factor for Graves’ orbitopathy: results of a cross-sectional study. Thyroid. 2018;28:386–94.
Bednarczuk T, Schomburg L. Challenges and perspectives of selenium supplementation in Graves’ disease and orbitopathy. Hormones (Athens). 2020;19:31–9.
Office of Dietary Supplements - Selenium. https://ods.od.nih.gov/factsheets/Selenium-Consumer/. .
Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. 2017;18. https://doi.org/10.3390/ijms18091949.
Mackawy AMH, Al-ayed BM, Al-rashidi BM. Vitamin D deficiency and its association with thyroid disease. Int J Health Sci (Qassim). 2013;7:267–75.
Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin D receptor and T cell function. Front Immunol. 2013;4. https://doi.org/10.3389/fimmu.2013.00148.
Heisel CJ, Riddering AL, Andrews CA, Kahana A. Serum vitamin D deficiency is an independent risk factor for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;36:17–20.
Zoumalan CI, Cockerham KP, Turbin RE, Volpe NJ, Kazim M, Douglas RS, et al. Efficacy of corticosteroids and external beam radiation in the management of moderate to severe thyroid eye disease. J Neuroophthalmol. 2007;27:205–14.
Young SM, Lim AYN, Lang SS, Lee KO, Sundar G. Efficacy and safety of pulsed intravenous methylprednisolone in early active thyroid eye disease. Orbit. 2019;38:362–9.
Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20.
Marcocci C, Bartalena L, Bogazzi F, Bruno-Bossio G, Lepri A, Pinchera A. Orbital radiotherapy combined with high dose systemic glucocorticoids for Graves’ ophthalmopathy is more effective than radiotherapy alone: results of a prospective randomized study. J Endocrinol Investig. 1991;14:853–60.
Strianese D. Efficacy and safety of immunosuppressive agents for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34:S56–9.
Tanikawa T, Okada Y, Tanaka Y. Intravenous cyclophosphamide pulse therapy is effective for refractory Graves’ ophthalmopathy. J UOEH. 2006;28:185–91.
Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154:511–7.
Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100:432–41.
Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31.
Shen W-C, Lee C-H, Loh E-W, Hsieh A-T, Chen L, Tam K-W. Efficacy and safety of rituximab for the treatment of Graves’ obitopathy: a meta-analysis of randomized controlled trials. Pharmacotherapy. 2018;38:503–10.
Savino G, Mandarà E, Gari M, Battendieri R, Corsello SM, Pontecorvi A. Intraorbital injection of rituximab versus high dose of systemic glucocorticoids in the treatment of thyroid-associated orbitopathy. Endocrine. 2015;48:241–7.
Li J, Xiao Z, Hu X, Li Y, Zhang X, Zhang S, et al. The efficacy of rituximab combined with 131I for ophthalmic outcomes of Graves’ ophthalmopathy patients. Pharmacology. 2017;99:144–52.
Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. 2008;18:959–65.
Jyonouchi SC, Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves’ ophthalmopathy. Thyroid. 2001;11:929–34.
Rajaii F, McCoy AN, Smith TJ. Cytokines are both villains and potential therapeutic targets in thyroid-associated ophthalmopathy: from bench to bedside. Expert Rev Ophthalmol. 2014;9:227–34.
Celik I, Akalin S, Erbaş T. Serum levels of interleukin 6 and tumor necrosis factor-alpha in hyperthyroid patients before and after propylthiouracil treatment. Eur J Endocrinol. 1995;132:668–72.
Molnár I, Balázs C. High circulating IL-6 level in Graves’ ophthalmopathy. Autoimmunity. 1997;25:91–6.
Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. J Clin Endocrinol Metab. 1996;81:2976–9.
Salvi M, Pedrazzoni M, Girasole G, Giuliani N, Minelli R, Wall JR, et al. Serum concentrations of proinflammatory cytokines in Graves’ disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol. 2000;143:197–202.
Pedro ABB, Romaldini JH, Takei K. Changes of serum cytokines in hyperthyroid Graves’ disease patients at diagnosis and during methimazole treatment. Neuroimmunomodulation. 2011;18:45–51.
Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010;20:59–65.
Raychaudhuri N, Douglas RS, Smith TJ. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PLoS One. 2010;5:e15296.
Drug Approval Package: ACTEMRA (tocilizumab). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/125276Orig1s114TOC.cfm. .
Copperman T, Idowu OO, Kersten RC, Vagefi MR. Subcutaneous tocilizumab for thyroid eye disease: simplified dosing and delivery. Ophthalmic Plast Reconstr Surg. 2019;35:e64–6.
Sy A, Eliasieh K, Silkiss RZ. Clinical response to tocilizumab in severe thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2017;33:e55–7.
Canas CA, Bonilla-Abadia F, Vallejo K, Rengifo HM, Gallon MA, Tobon GJ. Successful treatment for severe thyroid-associated ophthalmopathy with tocilizumab. Endocr Metab Immune Disord Drug Targets. 2018;18:665–7.
Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195:181–90.
Mohyi M, Smith TJ. IGF-I receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol. 2018;61:T29–43.
Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–405.
Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16:251–7.
Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–50.
Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–54.
Varewijck AJ, Boelen A, Lamberts SWJ, Fliers E, Hofland LJ, Wiersinga WM, et al. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013;98:769–76.
Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99:E1635–40.
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–52.
Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Rep. 2020;19:100744. https://doi.org/10.1016/j.ajoc.2020.100744.
Mackness BC, Jaworski JA, Boudanova E, Park A, Valente D, Mauriac C, et al. Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. MAbs. 2019;11:1276–88.
Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–9.
Pipeline. Immunovant.
Fong R, Collins J, Coquery C Targeting the neonatal Fc receptor for the treatment of moderate-to-severe active Graves’ ophthalmopathy. 1.
Collins J, Jones L, Snyder M, Sicard E, Griffin P, Webster L, Fong R, Coquery C, Piscitelli S IMVT-1401 (RVT-1401), A novel anti-FcRn monoclonal antibody, was well tolerated in healthy subjects and reduced serum IgG following subcutaneous or intravenous administration. 1.
Immunovant Sciences GmbH (2020) A phase 2a, multicenter, open-label study of RVT-1401 for the treatment of patients with moderate to severe active Graves’ ophthalmopathy. clinicaltrials.gov
Ramesh S, Nobori A, Wang Y, Rootman D, Goldberg RA. Orbital expansion in cranial vault after minimally invasive extradural transorbital decompression for thyroid orbitopathy. Ophthalmic Plast Reconstr Surg. 2019;35:17–21.
Leong SC, Karkos PD, Macewen CJ, White PS. A systematic review of outcomes following surgical decompression for dysthyroid orbitopathy. Laryngoscope. 2009;119:1106–15.
DeParis SW, Tian J, Rajaii F. Practice patterns in orbital decompression surgery among American Society of Ophthalmic Plastic and Reconstructive Surgery members. Ophthalmol Therapy. 2019;8:541–8.
Sellari-Franceschini S, Rocchi R, Marinò M, Bajraktari A, Mazzi B, Fiacchini G, et al. Rehabilitative orbital decompression for Graves’ orbitopathy: results of a randomized clinical trial. J Endocrinol Investig. 2018;41:1037–42.
Willaert R, Maly T, Ninclaus V, Huvenne W, Vermeersch H, Brusselaers N. Efficacy and complications of orbital fat decompression in Graves’ orbitopathy: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2020;49:496–504.
Jefferis JM, Jones RK, Currie ZI, Tan JH, Salvi SM. Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye (Lond). 2018;32:626–36.
Zhang S, Li Y, Wang Y, Zhong S, Liu X, Huang Y, et al. Comparison of rim-sparing versus rim-removal techniques in deep lateral wall orbital decompression for Graves’ orbitopathy. Int J Oral Maxillofac Surg. 2019;48:461–7.
Lu JE, Pfeiffer ML, Burnstine MA. Graded transantral orbital decompression outcomes in stable thyroid eye disease: a series of 47 orbits. Orbit. 2020:1–7.
Goldberg RA, Soroudi AE, McCann JD. Treatment of prominent eyes with orbital rim onlay implants: four-year experience. Ophthalmic Plast Reconstr Surg. 2003;19:38–45.
Mueller SK, Miyake MM, Lefebvre DR, Freitag SK, Bleier BS. Long-term impact of endoscopic orbital decompression on sinonasal-specific quality of life. Laryngoscope. 2018;128:785–8.
Tyler MA, Zhang CC, Saini AT, Yao WC. Cutting-edge endonasal surgical approaches to thyroid ophthalmopathy. Laryngoscope Investig Otolaryngol. 2018;3:100–4.
Cho RI, Choe CH, Elner VM. Ultrasonic bone removal versus high-speed burring for lateral orbital decompression: comparison of surgical outcomes for the treatment of thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2010;26:83–7.
Stähr K, Eckstein A, Holtmann L, Schlüter A, Dendy M, Lang S, et al. A comparative analysis of piezosurgery and oscillating saw for balanced orbital decompression. Orbit. 2019;38:433–9.
Naik MN, Nema A, Ali MH, Ali MJ. Piezoelectric surgery versus mechanical drilling for orbital floor decompression: effect on infraorbital hypoaesthesia. Orbit. 2019;38:184–6.
Grusha YO, Fedorov AA, Kolodina AS, Sviridenko NY. Comparative electron microscopy study of the bone surfaces relief after ultrasonic and mechanical high-speed bone removal in orbital decompression. Vestn oftalmol. 2019;135:155–9.
Costan VV, Ciocan-Pendefunda C-C, Ciofu ML, Boisteanu O, Timofte DV, Gheorghe L, et al. Balancing orbital volume reduction and redistribution for a tailored surgical treatment in Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:2313–20. https://doi.org/10.1007/s00417-020-04807-4.
Kitaguchi Y, Takahashi Y, Kakizaki H. Computed tomography-based prediction of exophthalmos reduction after deep lateral orbital wall decompression for Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257:2759–67.
Rajabi MT, Tabary M, Baharnoori S, Salabati M, Mahmoudzadeh R, Hosseinzadeh F, et al. Orbital anatomical parameters affecting outcome of deep lateral orbital wall decompression. Eur J Ophthalmol. 2020;1120672120941433.
Oeverhaus M, Copei A, Mattheis S, Ringelstein A, Tiemessen M, Esser J, et al. Influence of orbital morphology on proptosis reduction and ocular motility after decompression surgery in patients with Graves’ orbitopathy. PLoS One. 2019;14:e0218701.
Vaidya A, Kakizaki H, Takahashi Y. Changes in field of binocular single vision and ocular deviation angle after balanced orbital decompression in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;37:154–60. https://doi.org/10.1097/IOP.0000000000001712.
Susarla SM, Duncan K, Mahoney NR, Merbs SL, Grant MP. Virtual surgical planning for orbital reconstruction. Middle East Afr J Ophthalmol. 2015;22:442–6.
Sohrab M, Merbs SL, Grant MP, Mahoney NR. Accuracy of bone removal in orbital decompressions using preoperative planning and intraoperative navigation. Las Vegas: The American Society of Ophthalmic Plastic and Reconstructive Surgery Fall Symposium; 2015.
Spalthoff S, Jehn P, Zimmerer R, Rana M, Gellrich N-C, Dittmann J. Modified lateral orbital wall decompression in Graves’ orbitopathy using computer-assisted planning. Int J Oral Maxillofac Surg. 2018;47:167–74.
Heisel CJ, Tuohy MM, Riddering AL, Sha C, Kahana A. Stereotactic navigation improves outcomes of orbital decompression surgery for thyroid associated orbitopathy. Ophthalmic Plast Reconstr Surg. 2020;36:553–6. https://doi.org/10.1097/IOP.0000000000001630.
Lewis KT, Bullock JR, Drumright RT, Olsen MJ, Penman AD. Changes in peripapillary blood vessel density in Graves’ orbitopathy after orbital decompression surgery as measured by optical coherence tomography angiography. Orbit. 2019;38:87–94.
Yoo TK, Choi JY, Kim HK. A generative adversarial network approach to predicting postoperative appearance after orbital decompression surgery for thyroid eye disease. Comput Biol Med. 2020;118:103628.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nicholas Mahoney served on an advisory board for Horizon Therapeutics in June 2020.
Fatemeh Rajaii has worked a consultant for Horizon Therapeutics.
Fatemeh Rajaii is supported by the National Eye Institute of the National Institutes of Health under award number K08EY027093.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurologic Ophthalmology and Otology
Rights and permissions
About this article
Cite this article
Mahoney, N.R., Rajaii, F. Current Management of Thyroid Eye Disease. Curr Treat Options Neurol 23, 21 (2021). https://doi.org/10.1007/s11940-021-00675-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11940-021-00675-3