Abstract
Purpose of Review
Prostate ablation is increasingly being utilized for the management of localized prostate cancer. There are several energy modalities with varying mechanism of actions which are currently used for prostate ablation. Prostate ablations, whether focal or whole gland, are performed under ultrasound and/or MRI guidance for appropriate treatment plan execution and monitoring. A familiarity with different intraoperative imaging findings and expected tissue response to these ablative modalities is paramount. In this review, we discuss the intraoperative, early, and delayed imaging findings in prostate from the effects of prostate ablation.
Recent Findings
The monitoring of ablation both during and after the therapy became increasingly important due to the precise targeting of the target tissue. Recent findings suggest that real-time imaging techniques such as MRI or ultrasound can provide anatomical and functional information, allowing for precise ablation of the targeted tissue and increasing the effectiveness and precision of prostate cancer treatment. While intraprocedural imaging findings are variable, the follow-up imaging demonstrates similar findings across various energy modalities.
Summary
MRI and ultrasound are two of the frequently used imaging techniques for intraoperative monitoring and temperature mapping of important surrounding structures. Follow-up imaging can provide valuable information about ablated tissue, including the success of the ablation, presence of residual cancer or recurrence after the ablation. It is critical and helpful to understand the imaging findings during the procedure and at different follow-up time periods to evaluate the procedure and its outcome.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Li Q, Xiang F, Lin X, Grajo JR, Yang L, Xu Y, et al. The role of imaging in prostate cancer care pathway: novel approaches to urologic management challenges along 10 imaging touch points. Urology. 2018;01(119):23–31.
Calio B, Kasson M, Sugano D, Ortman M, Gaitonde K, Verma S, et al. Multiparametric MRI: an opportunity for focal therapy of prostate cancer. Semin Roentgenol. 2018;53(3):227–33.
Berends J, Dupati A, Dibianco J, George AK. Focal therapy is a viable treatment for low-risk prostate cancer. J Endourol. 2021;35(9):1281–3.
Hayes M, Lin-Brande M, Isharwal S. Primary focal therapy for localized prostate cancer: a review of the literature. Oncology (Williston Park). 2021;35(5):261–8.
Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol. 2019;31(3):200–6.
Connor MJ, Gorin MA, Ahmed HU, Nigam R. Focal therapy for localized prostate cancer in the era of routine multi-parametric MRI. Prostate Cancer Prostatic Dis. 2020;23(2):232–43.
Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13(11):641–53.
Beyer LP, Wiggermann P. Treatment planning, needle insertion, image guidance, and endpoint assessment. Irreversible Electroporation in Clinical Practice: Springer; 2018. p. 115–120.
Zhang X, Landgraf L, Bailis N, Unger M, Jochimsen TH, Melzer A. Image-guided high-intensity focused ultrasound, a novel application for interventional nuclear medicine? J Nucl Med. 2021;62(9):1181–8.
• Napoli A, Alfieri G, Scipione R, Leonardi A, Fierro D, Panebianco V, et al. High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices. 2020;17(5):427–33. This paper highlights the importance of focal therapy of prostate cancer and review HIFU as a frequently used treatment method. It thoroughly explains the procedures, the mechanism of action, candidate, the potential risks and side effects, and clinical outcomes. Given the increasing use and advancement of focal therapy in treating prostate cancer, this article provides a comprehensive understanding of the HIFU procedure.
Chapelon J, Rouvière O, Crouzet S, Gelet A. Prostate focused ultrasound therapy. Ther Ultrasound. 2016:21–41.
Seggie DA, Doherty GM, Leeman S, Ferrari LA. Ultrasonic imaging using the instantaneous frequency of pulse-echo signals. Acoust Imaging. 1985:487–496.
Halliwell M. A tutorial on ultrasonic physics and imaging techniques. Proc Inst Mech Eng Part H J Eng Med. 2010;224(2):127–42.
Senneville BDD, Moonen C, Ries M. MRI-guided HIFU methods for the ablation of liver and renal cancers. Ther Ultrasound. 2016:43–63.
Chen WH, Sanghvi NT, Carlson R, Uchida T. Real‐time tissue change monitoring on the Sonablate® 500 during high intensity focused ultrasound (HIFU) treatment of prostate cancer. AIP Conf Proc. 2011;1359(1):391–396. American Institute of Physics
Chen WH, Sanghvi NT, Carlson R, Schatzl G, Marberger M. Validation of tissue change monitoring (TCM) on the Sonablate® 500 during high intensity focused ultrasound (HIFU) treatment of prostate cancer with real-time thermometry. In AIP Conf Proc. 2012;1481(1):53–58. American Institute of Physics.
Shoji S, Koizumi N, Yuzuriha S, Kano T, Ogawa T, Nakano M, et al. Development and future prospective of treatment for localized prostate cancer with high-intensity focused ultrasound. J Med Ultrason. 2022.
Yee CH, Chiu PK, Teoh JY, Ng CF, Chan CK, Hou SM. High-intensity focused ultrasound (HIFU) focal therapy for localized prostate cancer with MRI-US fusion platform. Adv Urol. 2021;14(2021):7157973.
Shoji S, Hiraiwa S, Uemura K, Nitta M, Hasegawa M, Kawamura Y, et al. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol. 2020;25(10):1844–53.
•• Bui T, Glavis-Bloom J, Chahine C, Mehta R, Wolfe T, Bhatter P, et al. Prostate minimally invasive procedures: complications and normal vs. abnormal findings on multiparametric magnetic resonance imaging (mpMRI). Abdom Radiol. 2021;46(9):4388–4400. This article holds significant importance and serves a valuable purpose. It gives insight into focal therapy modalities of prostate cancer in addition to investigating the impact of mpMRI in evaluating and detecting complications and abnormal findings after focal therapy of the prostate. The study emphasizes the importance of using mpMRI to guide clinical decisions and improve patient outcomes and highlights the need for standardized protocols for interpreting and reporting mpMRI findings in these patients.
Kirkham AP, Emberton M, Hoh IM, Illing RO, Freeman AA, Allen C. MR imaging of prostate after treatment with high-intensity focused ultrasound. Radiology. 2008;246(3):833–44.
Apfelbeck M, Chaloupka M, Schlenker B, Stief CG, Clevert DA. Follow-up after focal therapy of the prostate with high intensity focused ultrasound (HIFU) using contrast enhanced ultrasound (CEUS) in combination with MRI image fusion. Clin Hemorheol Microcirc. 2019;73(1):135–43.
Muller BG, Van den Bos W, Brausi M, Fütterer JJ, Ghai S, Pinto PA, et al. Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol. 2015;33:1503–9.
Huang H, Zhu ZQ, Zhou ZG, Chen LS, Zhao M, Zhang Y, et al. Contrast-enhanced transrectal ultrasound for prediction of prostate cancer aggressiveness: the role of normal peripheral zone time-intensity curves. Sci Rep. 2016;08(6):38643.
de Castro Abreu AL, Ashrafi AN, Gill IS, Oishi M, Winter MW, Park D, et al. Contrast-enhanced transrectal ultrasound for follow-up after focal HIFU ablation for prostate cancer. J Ultrasound Med. 2019;38(3):811–9.
Apfelbeck M, Clevert DA, Ricke J, Stief C, Schlenker B. Contrast enhanced ultrasound (CEUS) with MRI image fusion for monitoring focal therapy of prostate cancer with high intensity focused ultrasound (HIFU)1. Clin Hemorheol Microcirc. 2018;69(1–2):93–100.
Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Prostate cancer: state of the art imaging and focal treatment. Clin Radiol. 2017;72(8):665–79.
Siedek F, Yeo SY, Heijman E, Grinstein O, Bratke G, Heneweer C, Puesken M, Persigehl T, Maintz D, Grüll H. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU): technical background and overview of current clinical applications (part 1). InRöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 2019;191(6):522–530. © Georg Thieme Verlag KG.
Mihcin S, Melzer A. Principles of focused ultrasound. Minim Invasive Ther Allied Technol. 2018;27(1):41–50.
Ghai S, Louis AS, Van Vliet M, Lindner U, Haider MA, Hlasny E, et al. Real-time MRI-guided focused ultrasound for focal therapy of locally confined low-risk prostate cancer: feasibility and preliminary outcomes. Am J Roentgenol. 2015;205(2):W177–84.
• Wimper Y, Futterer JJ, Bomers JGR. MR imaging in real time guiding of therapies in prostate cancer. Life (Basel). 2022;12(2). https://doi.org/10.3390/life12020302. This article highlights the importance of using MRI as a monitoring and therapeutic technique for prostate cancer and offers insightful information on the most recent advancements in this area. The authors discuss the advantages and limitations of different techniques and emphasize the need for personalized treatment plans for prostate cancer patients, which can only be achieved through the use of advanced imaging techniques like MRI.
Masoom SN, Sundaram KM, Ghanouni P, Futterer J, Oto A, Ayyagari R, et al. Real-time MRI-guided prostate interventions. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14081860.
Boyes A, Tang K, Yaffe M, Sugar L, Chopra R, Bronskill M. Prostate tissue analysis immediately following magnetic resonance imaging guided transurethral ultrasound thermal therapy. J Urol. 2007;178(3 Pt 1):1080–5.
Larson BT, Collins JM, Huidobro C, Corica A, Vallejo S, Bostwick DG. Gadolinium-enhanced MRI in the evaluation of minimally invasive treatments of the prostate: correlation with histopathologic findings. Urology. 2003;62(5):900–4.
Lodeizen O, de Bruin M, Eggener S, Crouzet S, Ghai S, Varkarakis I, et al. Ablation energies for focal treatment of prostate cancer. World J Urol. 2019;37(3):409–18.
Shah TT, Ahmed H, Kanthabalan A, Lau B, Ghei M, Maraj B, et al. Focal cryotherapy of localized prostate cancer: a systematic review of the literature. Expert Rev Anticancer Ther. 2014;14(11):1337–47.
Nguyen HD, Allen BJ, Pow-Sang JM. Focal cryotherapy in the treatment of localized prostate cancer. Cancer Control. 2013;20(3):177–80.
Bermejo CE, Pisters LL. Cryotherapy for prostate cancer. Expert Rev Anticancer Ther. 2003;3(3):393–401.
Lau B, Shah TT, Valerio M, Hamid S, Ahmed HU, Arya M. Technological aspects of delivering cryotherapy for prostate cancer. Expert Rev Med Devices. 2015;12(2):183–90.
Gangi A, Tsoumakidou G, Abdelli O, Buy X, de Mathelin M, Jacqmin D, et al. Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol. 2012;22(8):1829–35.
Cornud F, Bomers J, Futterer JJ, Ghai S, Reijnen JS, Tempany C. MR imaging-guided prostate interventional imaging: ready for a clinical use? Diagn Interv Imaging. 2018;99(11):743–53.
Mathew MS, Oto A. MR imaging-guided focal therapies of prostate cancer. Magn Reson Imaging Clin N Am. 2019;27(1):131–8.
Onik G. Image-guided prostate cryosurgery: state of the art. Cancer Control. 2001;8(6):522–31.
Overduin CG, Bomers JG, Jenniskens SF, Hoes MF, Ten Haken B, de Lange F, et al. T1-weighted MR image contrast around a cryoablation iceball: a phantom study and initial comparison with in vivo findings. Med Phys. 2014;41(11):112301.
Gowardhan B, Greene D. Cryotherapy for the prostate: an in vitro and clinical study of two new developments; advanced cryoneedles and a temperature monitoring system. BJU Int. 2007;100(2):295–302.
Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: modalities, findings and future considerations. Nat Rev Urol. 2010;7(10):562–71.
Kalbhen CL, Hricak H, Shinohara K, Chen M, Parivar F, Kurhanewicz J, et al. Prostate carcinoma: MR imaging findings after cryosurgery. Radiology. 1996;198(3):807–11.
Natarajan S, Jones TA, Priester AM, Geoghegan R, Lieu P, Delfin M, et al. Focal laser ablation of prostate cancer: feasibility of magnetic resonance imaging-ultrasound fusion for guidance. J Urol. 2017;198(4):839–47.
Werntz RP, Eggener SE. Novel focal therapy treatment options for prostate cancer. Curr Opin Urol. 2018;28(2):178–83.
Eymerit-Morin C, Zidane M, Lebdai S, Triau S, Azzouzi AR, Rousselet MC. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch. 2013;463(4):547–52.
Westin C, Chatterjee A, Ku E, Yousuf A, Wang S, Thomas S, et al. MRI findings after MRI-guided focal laser ablation of prostate cancer. AJR Am J Roentgenol. 2018;211(3):595–604.
Gaur S, Turkbey B. Prostate MR imaging for posttreatment evaluation and recurrence. Radiol Clin North Am. 2018;56(2):263–75.
Coleman JA, Scardino PT. Targeted prostate cancer ablation: energy options. Curr Opin Urol. 2013;23(2):123–8.
Ting F, Tran M, Böhm M, Siriwardana A, Van Leeuwen PJ, Haynes AM, et al. Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis. 2016;19(1):46–52.
Rubinsky L, Guenther E, Mikus P, Stehling M, Rubinsky B. Electrolytic effects during tissue ablation by electroporation. Technol Cancer Res Treat. 2016;15(5):NP95–103.
Scheltema MJ, Postema AW, de Bruin DM, Buijs M, Engelbrecht MR, Laguna MP, et al. Irreversible electroporation for the treatment of localized prostate cancer: a summary of imaging findings and treatment feedback. Diagn Interv Radiol. 2017;23(5):365.
Beyer LP, Pregler B, Verloh N, Brünn K, Haimerl M, Stroszczynski C, et al. Effect of irreversible electroporation of prostate cancer on microcirculation: imaging findings in contrast-enhanced T1-weighted 3D MRI. Clin Hemorheol Microcirc. 2017;67(3–4):399–405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there was no conflict of interest with this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Examples of real-time HIFU transrectal ultrasound images. A–B shows the procedure development and changes during the procedure. The ablation field is marked as a blue band and the site of ablation is orange. The popcorn effect is seen as a hyperechoic area in the region of the ablation (green circle). C–D Shows the corresponding axial view of A and B
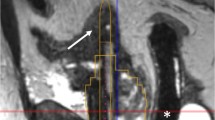
HIFU. pre-treatment T2 MRI (A), pre-treatment ADC MRI (B) demonstrating a lesion in the left apical posterior peripheral zone (white arrows), biopsied as 3 + 4 prostate cancer. 6-month post-treatment T2 MRI (C) and ADC MRI (D) demonstrating tissue changes post-treatment including volume loss, fibrosis, and capsular retraction (red arrows)
Contrast-enhanced transrectal ultrasound. A A pre-treatment split-screen grayscale image and B a pre-treatment contrast-enhanced transrectal ultrasound image showing a lesion in the left lobe of the prostate gland with increased enhancement (white arrow). C An immediate post-HIFU split-screen grayscale image and D a post-HIFU contrast-enhanced transrectal ultrasound image showing post-treatment changes in the ablation area as a non-enhancing area (white arrow). Figure adapted with permission from reference [25] (reused with permission from Abreu, Journal of Ultrasound in Medicine, published by Springer, 2018, License number: 5477200383530)
Transrectal ultrasound ablation (TULSA). A MRI real-time thermometry images of the prostate during TULSA shows the real-time temperature of the different parts of the gland during ablation with the maximum temperature at the site of the ablation. B MRI T1 images of the prostate show left hemiablation of the prostate gland with a non-enhancing area on the left side of the prostate indicating ablation necrosis. Figure adapted with permission from reference [31•] (Wimper, journal of MDPI, published by Life, 2022)
Examples of cryotherapy images. A shows the prostate gland with hyperechoic lines manifesting cryoneedles inside the gland (blue arrows). B–C are pictures of real-time cryotherapy showing the edge of the iceball seen as a hyperechoic thin line (red arrows) with the posterior shadow (hypoechoic area)
Cryotherapy. Pre-treatment prostate MRI T2 (A) and DWI (B) demonstrating a lesion in the right anterior paramedian prostate (white arrow). Post-treatment MRI T2 (C) and DWI (D) demonstrating tissue changes post-treatment (red arrows) including prostate atrophy, fibrosis, and capsular retraction with no diffusion restriction in the area of the ablation
Examples of IRE images. A During the ablation. Hyperechoic areas around the electrodes during ablation; (electrodes marked by red circles were active during this image capture) B post ablation image. Area of hyperechogencity in the ablation zone and obscuration of prostate anterior to the ablation zone
Irreversible electroporation. Pre-treatment prostate MRI T2 (A) and DWI (B) demonstrating PIRADS 5 lesion in the left mid-anterior transitional zone (white arrows). Post-treatment prostate MRI T2 (C), DWI (D), and ADC (E) demonstrating post-treatment necrosis (yellow arrows). There is a restricted diffusion on DWI (D) and mild enhancement on DCE (E), concerning possible residual disease (red arrows)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tayebi, S., Verma, S. & Sidana, A. Real-Time and Delayed Imaging of Tissue and Effects of Prostate Tissue Ablation. Curr Urol Rep 24, 477–489 (2023). https://doi.org/10.1007/s11934-023-01175-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11934-023-01175-4